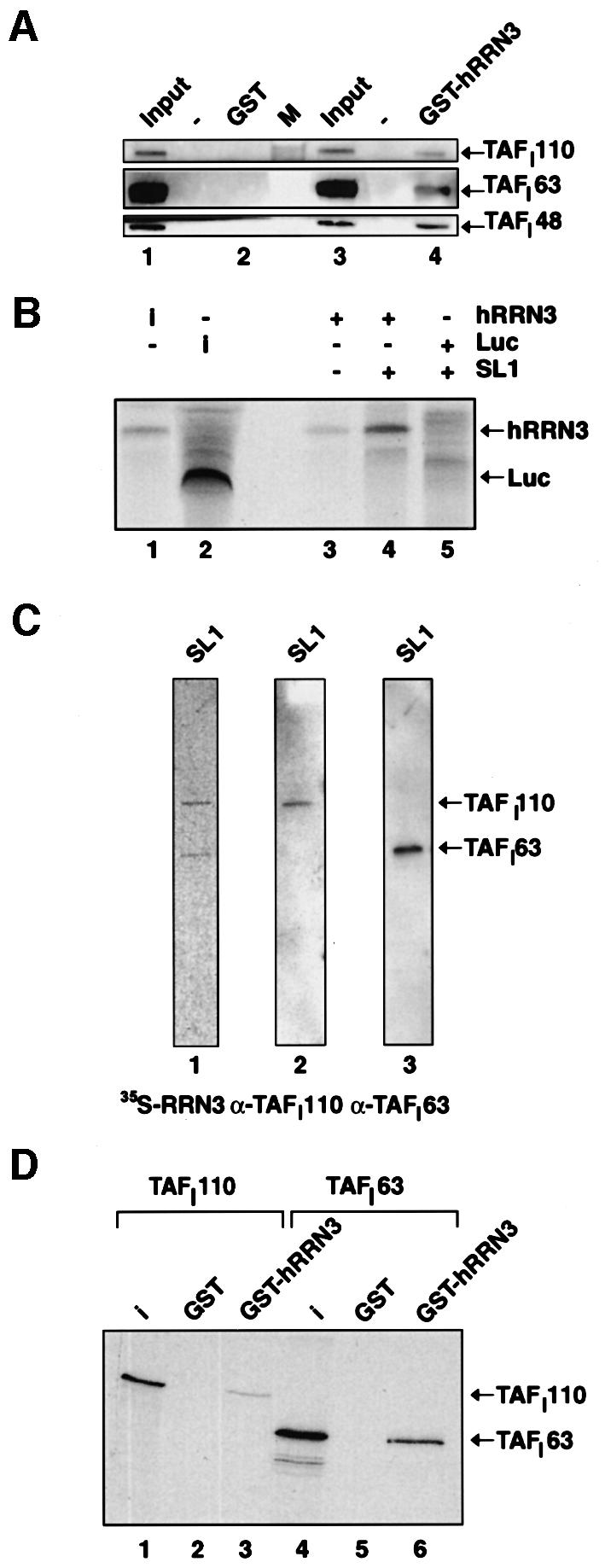
Fig. 4. hRRN3 interacts with SL1. (A) Highly purified SL1 (see Materials and methods) specifically interacts with recombinant and purified GST–hRRN3, as revealed by immunoblotting of the relevant strips of the immunoblot with antibodies specific for three subunits of SL1, TAFI110, TAFI63 and TAFI48. (B) hRRN3 interacts with SL1, which had been immunoprecipitated with antibodies specific for TBP. FLAG-epitope affinity-purified, 35S-radiolabelled hRRN3 (10% of input in lane 1) and luciferase (10% of input, lane 2) were incubated with SL1 immobilized via a TBP antibody to protein G–Sepharose beads (lanes 4 and 5). As an additional control, hRRN3 was added to antibody-loaded beads without SL1 (lane 3). Bound proteins were subjected to SDS–PAGE. The gel was fixed, dried and subjected to autoradiography. (C) FLAG-tag affinity-purified [35S]hRRN3 specifically interacts with two subunits of SL1, TAFI110 and TAFI63 in a far-western blot of highly purified SL1 (lane 1). The blot was probed with antibodies specific for TAFI110 (lane 2) and TAFI63 (lane 3), confirming their identity. (D) GST–hRRN3 interacts with two subunits of SL1. GST (lane 2 and 5) and GST–hRRN3 (lanes 3 and 6) on glutathione beads were incubated with in vitro translated [35S]methionine-labelled TAFI110 and TAFI63, and after extensive washing the resulting protein complexes were resolved by SDS–PAGE and autoradiography. Ten per cent of the TAFI110 and TAFI63 inputs are shown in lanes 1 and 4, respectively.
