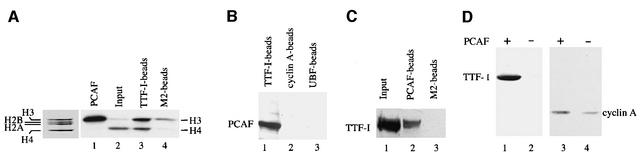
Fig. 1. TTF-I interacts with PCAF. (A) Pull-down of cellular HAT activity by TTF-I. A 10 µg aliquot of FLAG-TTF-I bound to 10 µl of M2-agarose beads (lane 3) or M2 beads saturated with the FLAG peptide (lane 4) was incubated with 2 mg of mouse whole-cell extract proteins for 4 h at 4°C in a total volume of 380 µl. After stringent washing, 50% of the beads were assayed for HAT activity using 5 µg of histones and 1 µCi of [3H]acetyl-CoA. In lanes 1 and 2, histone acetylation by recombinant PCAF or cell extract is shown. Histones were separated by 15% SDS–PAGE and visualized by Coomassie Blue staining (left panel) and fluorography (lanes 1–4). (B) Interaction of PCAF with bead-bound TTF-I. A 35 µl aliquot of Ni2+-NTA–agarose saturated with histidine-tagged TTF-I (lane 1) or cyclin A (lane 2), and M2-agarose saturated with FLAG-UBF (lane 3) were incubated with extracts from mouse cells. After washing, bead-bound proteins were subjected to western blot analysis using anti-PCAF antibodies. (C) Association of cellular TTF-I with PCAF. Bead-bound FLAG-PCAF (lane 2) or control beads (lane 3) were incubated with extract from mouse cells, and associated TTF-I was identified on immunoblots. In lane 1, the amount of TTF-I present in 10% of the extract is shown. (D) Co-immunoprecipitation of TTF-I and PCAF. Histidine-tagged TTF-I or cyclin A was co-expressed with FLAG-PCAF in Sf9 cells. PCAF was precipitated with anti-FLAG antibodies (M2) and analyzed on western blots for the presence of TTF-I (lanes 1 and 2) or cyclin A (lanes 3 and 4).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.