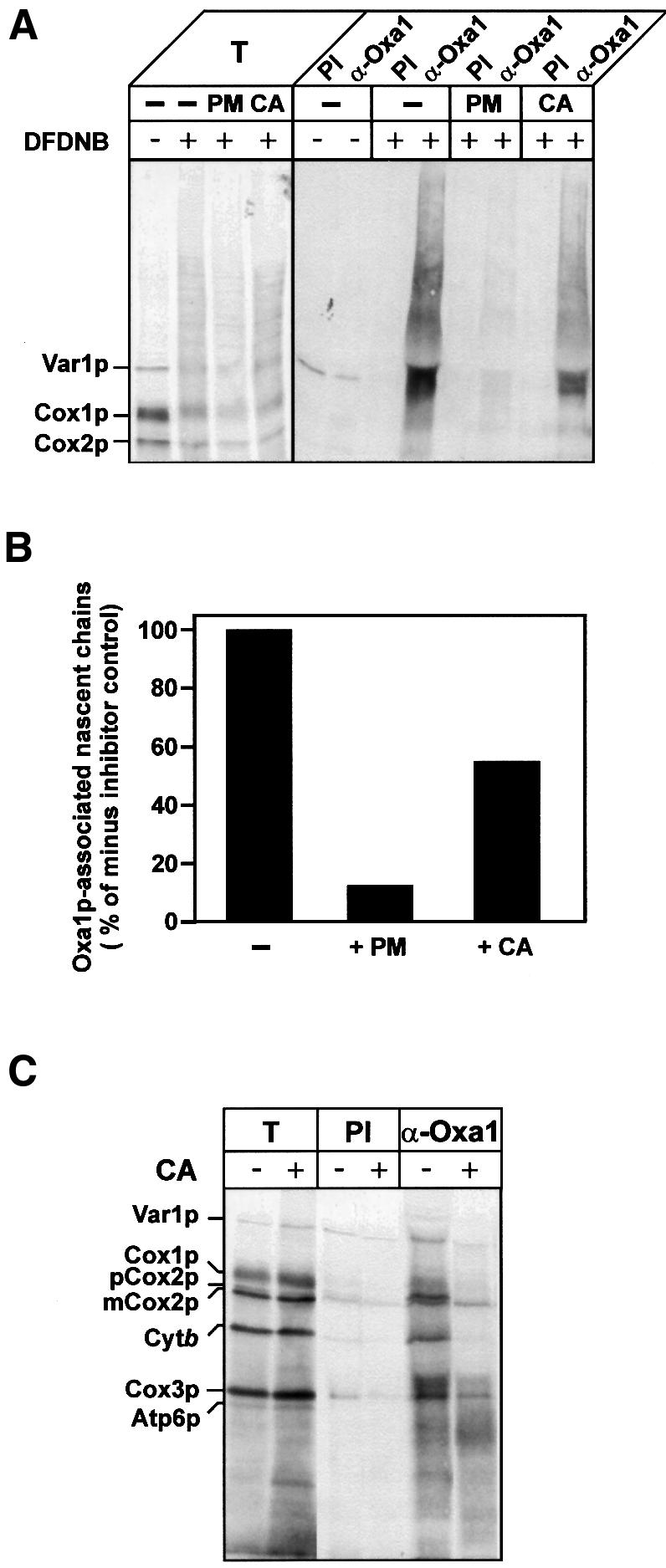
Fig. 4. Oxa1p interacts with mitochondrially encoded proteins predominantly during synthesis. (A and B) Translation in isolated wild-type mitochondria was performed in the presence of [35S]methionine, for 10 min at 25°C. Samples then received either puromycin (PM, 45 µg/ml) or chloramphenicol (CA, 3 mg/ml) or no addition (–), for 3 min at 25°C. The samples were incubated for a further 15 min, in the presence or absence of DFDNB (0.4 mM). Cross-linking and translation were stopped by the addition of 100 mM glycine pH 8.0 and 50 µg/ml puromycin. Re-isolated and washed mitochondria were lysed, and immunoprecipitation with either pre-immune serum (PI) or antiserum specific for Oxa1p (α-Oxa1) was performed. Immunoprecipitates were analyzed by SDS–PAGE and autoradiography (A). T, 0.5% of cross-linking reaction that was used for immunoprecipitation. (B) Quantification of the autoradiography. The total translation signals for each sample were initially quantified and were as follows (arbitrary units): no-inhibitor control, 156; PM-treated, 100; CA-treated, 150. The level of nascent chains associated with Oxa1p was then expressed as a percentage of the total translation signal for each of the treatments. The resulting value obtained in the absence of a translational inhibitor (control) was set to 100%, and the PM- and CA-treated values were then expressed as a percentage of the control. (C) Translation in isolated wild-type mitochondria was performed in the presence of [35S]methionine for 20 min at 25°C. Samples were placed on ice for 5 min. Aliquots were either treated with chloramphenicol (+CA, 3 mg/ml) or mock-treated (–CA). Following the addition of SPDP (0.3 mM), the samples were further incubated for 15 min at 25°C. The samples were processed and analyzed as described in Figure 2. T, 5% of cross-linking reaction that was used for immunoprecipitation.
