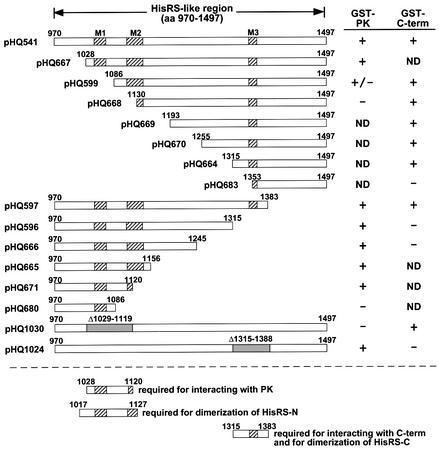
Fig. 6. Mapping of amino acids in the HisRS-N and HisRS-C regions required for interaction with the PK and C-term domains in vitro. The rectangular boxes depict different segments of the HisRS-like region that were synthesized in vitro in the presence of [35S]methionine, using the plasmids listed to the left of the boxes. The gray segments in the pHQ1030 and pHQ1024 constructs depict deleted residues. 35S-labeled proteins were incubated with GST, GST–PK(568–998) or GST–C-term(1498–1659) immobilized on glutathione–Sepharose beads. After extensive washing, the proteins bound to the beads were resolved by SDS–PAGE and visualized by fluorography, all as described above. None of these radiolabeled proteins bound to GST alone (data not shown). Binding (+) or failure to bind (–) to GST–PK or GST–C-term is indicated on the right under the columns headed ‘GST–PK’ or ‘GST–C-term’, respectively. ND, not determined. The rectangular boxes under the dashed line depict the GCN2 residues required for binding of HisRS-N to PK, for dimerization of HisRS-C and for its binding to the C-term, and for dimerization of HisRS-N, deduced from results in this figure and in Figures 2 and 3. The hatched boxes indicate the locations of conserved motifs M1–3.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.