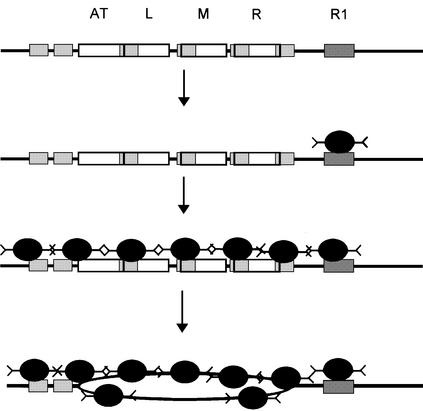
Fig. 5. A model describing the DnaA-dependent unwinding reaction at oriC. The DnaA box R1 and the three 13mers are labeled. The six ATP-DnaA boxes are indicated by small gray boxes. Initially, DnaA protein binds with a high affinity to DnaA box R1. This complex serves as an anchor for cooperative binding of ATP-DnaA to the ATP-DnaA boxes, positioning the protein to the region of unwinding. The formation of this complex needs high concentrations of ATP-DnaA protein and can be considered, therefore, as the rate-limiting reaction in unwinding. Single-stranded DNA resulting from unwinding will then be stabilized by cooperative binding of ATP-DnaA. The affinity of ATP-DnaA is higher to single-stranded ATP-DnaA boxes than to double-stranded ATP-DnaA boxes.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.