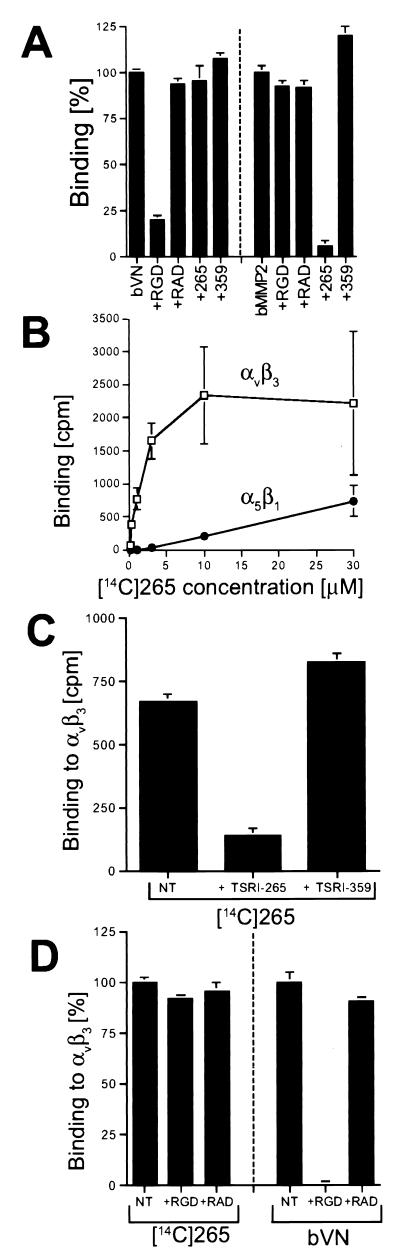
Figure 2.
TSRI265 binds directly to integrin αvβ3, suppressing the interaction between MMP2 and integrin αvβ3. (A) TSRI265 specifically blocks integrin αvβ3 binding to MMP2 without affecting interaction of αvβ3 with its classical ligand, vitronectin. Solid phase receptor binding of biotinylated MMP2 (bMMP2) or bVN to integrin αvβ3 was performed in the presence or absence of 3 μM TSRI265/TSRI359 or 100 μM cyclic RGD or RAD peptide. Binding was determined colorimetrically with an HRP-conjugated antibiotin mAb as described in the Materials and Methods. (B–D) TSRI265 binds specifically and saturably to integrin αvβ3 in an RGD-independent manner. Purified integrins αvβ3 and α5β1 were coated onto microtiter wells, which were subsequently blocked and incubated with 14C-labeled TSRI265 alone (B) or in the presence or absence of a 25-fold molar excess of unlabeled TSRI265 or TSRI359 (C) or 100 μM cyclic RGD or RAD peptide (D). bVN was used as a control and detected colorimetrically with an HRP-conjugated antibiotin mAb as described in Materials and Methods.