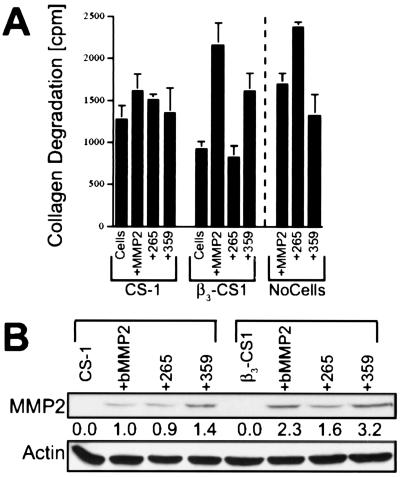
Figure 3.
TSRI265 suppresses cell-mediated collagenolytic activity by interfering with MMP2 binding to integrin αvβ3. (A) TSRI265 blocks cell-mediated β3-dependent utilization of active MMP2 to degrade collagen IV. CS-1 melanoma cells or CS-1 cells transfected with integrin β3 (β3CS-1) were incubated with active MMP2 in the presence or absence of either 10 μM TSRI265 or TSRI359, washed, and plated onto wells coated with [3H]collagen IV. As a control, active MMP2 was examined in the absence of cells (denoted by dotted line). After 36 h, a sample of media was removed and quantitated in a liquid scintillation counter. (B) TSRI265 blocks MMP2 binding to integrin αvβ3 on the cell surface. CS-1 or β3CS-1 cells were incubated with biotinylated MMP2 in the presence or absence of either 10 μM TSRI265 or TSRI359, washed twice, and lysed for analysis by SDS/PAGE and immunoblotting with an HRP-conjugated antibiotin mAb. Cells that had not been treated with bMMP2 are shown for comparison. Biotin-reactive bands corresponding to MMP2 were analyzed by scanning densitometry, and the relative intensities are shown under each lane. The blot was reprobed with an antiactin mAb to ensure equal loading of lysates.