Abstract
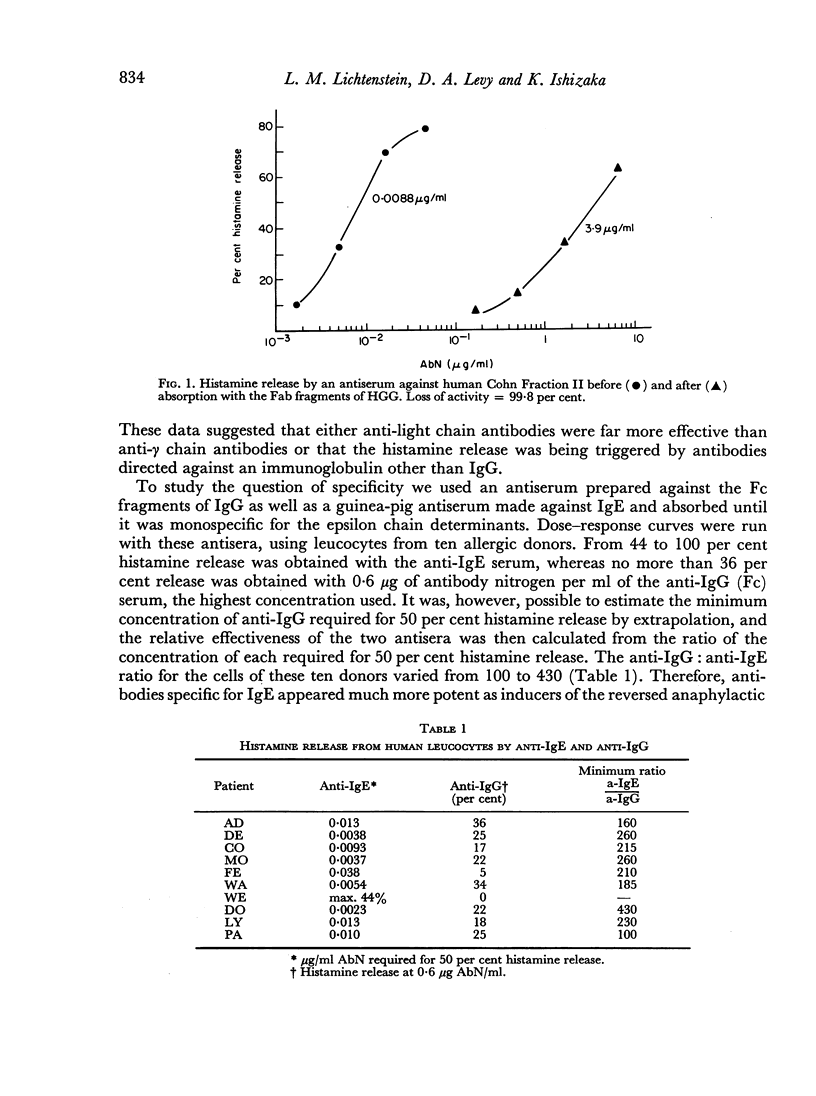
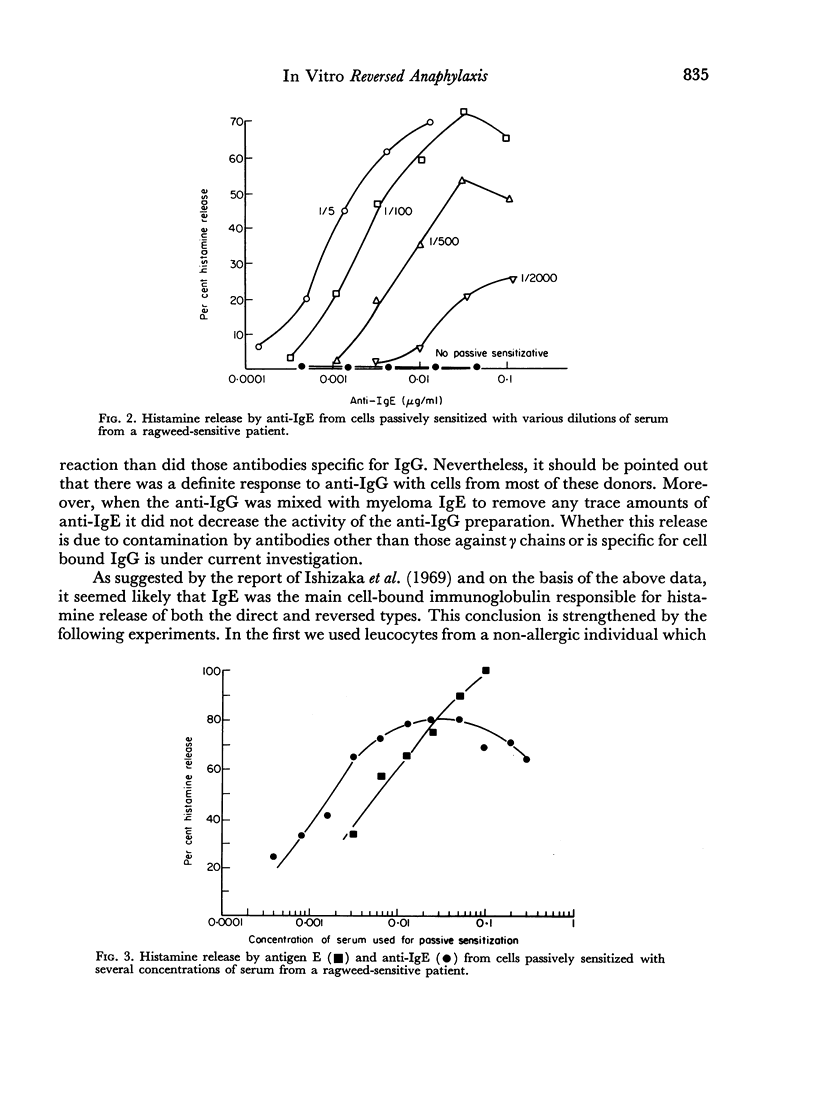
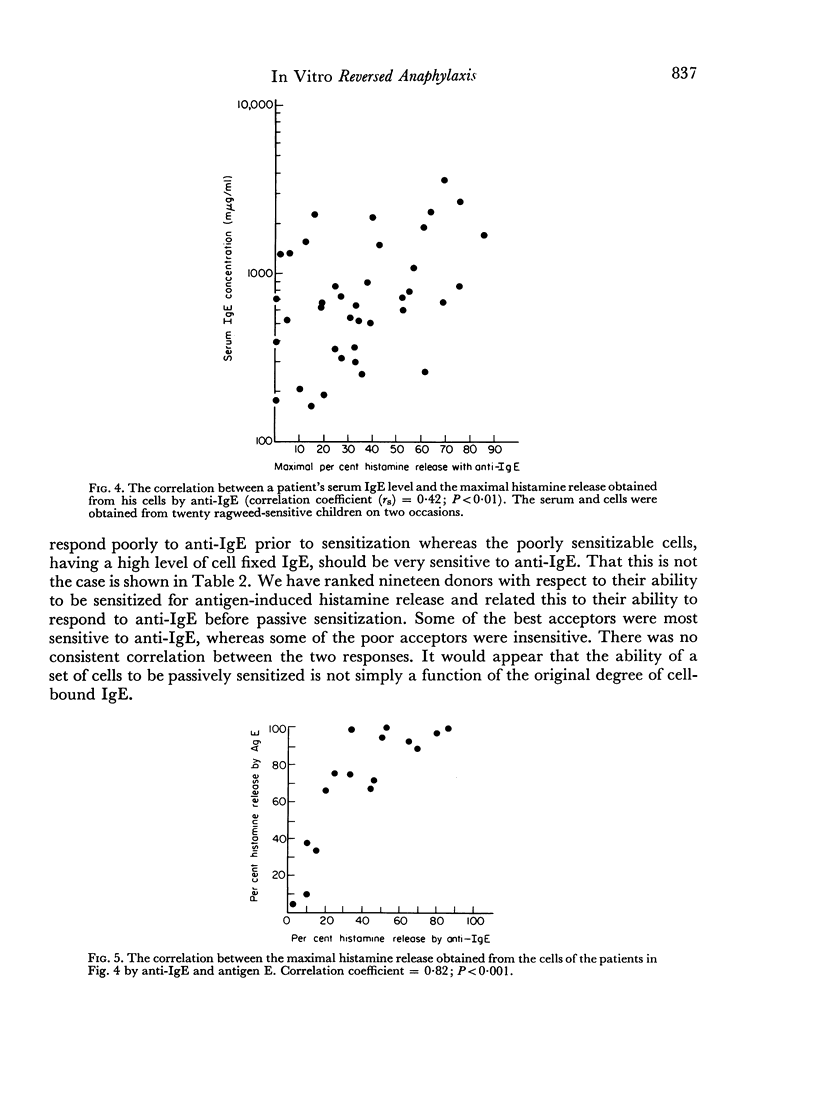
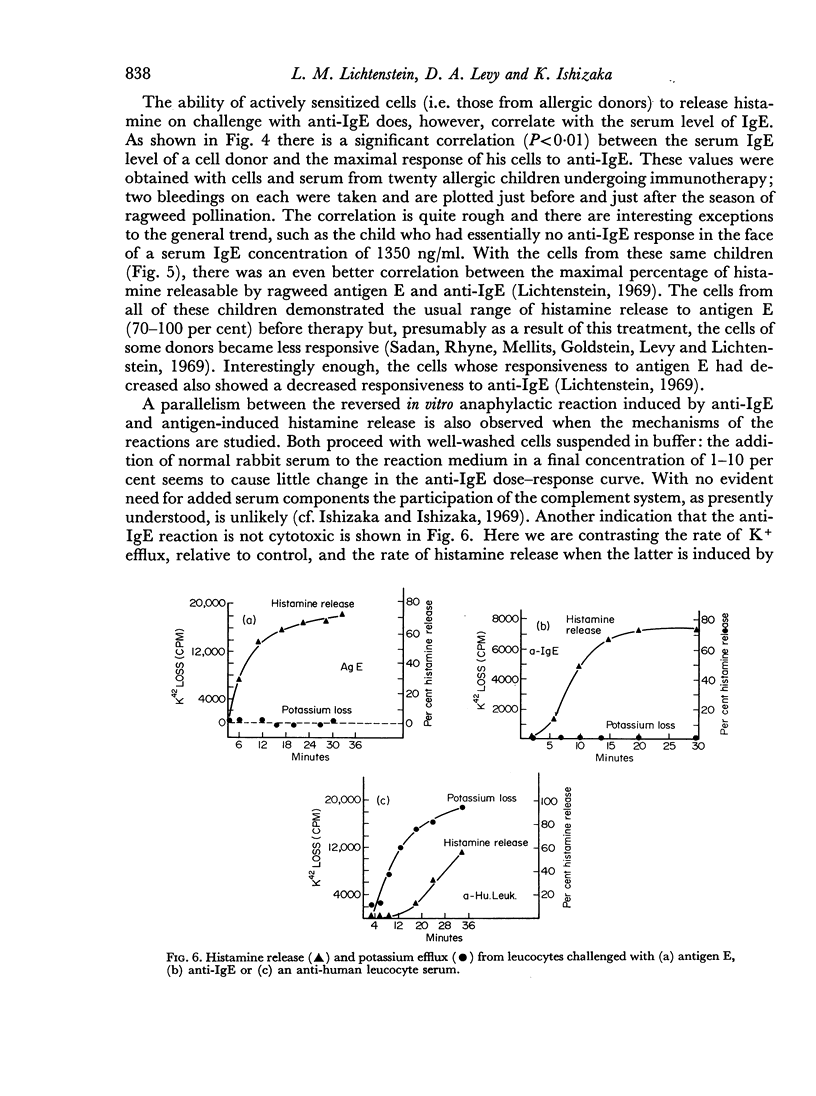
Leucocytes from allergic and most normal human donors release histamine when challenged with antibodies against human γ-globulin. This reaction (reversed in vitro anaphylaxis) is due primarily to anti-IgE antibodies although there is some response in most donors to antisera against IgG even after it has been absorbed with light and ε chains. The anti-IgE is, however, several 100-fold more potent than the anti-IgG. By passive sensitization of the leucocytes of a normal donor with serum from a ragweed-allergic patient it was shown that the normal cells became sensitive to anti-IgE and ragweed antigen E at the same time; in both cases, there was an inverse relationship between the serum concentration used for passive sensitization and the concentration of antigen or antibody required for histamine release. There is a rough correlation (rs = 0.42; P<0.01) between the serum IgE concentration and the response of leucocytes from allergic donors to anti-IgE and an excellent correlation (rs = 0.82; P<0.01) between the response of the cells to ragweed antigen E and anti-IgE. There is also a strong parallel between the mechanism of direct, antigen mediated histamine release and the reversed reaction induced by anti-IgE. Both appear to be non-serum requiring, non-cytotoxic, secretory-like responses which are inhibited by theophylline, cyclic AMP and colchicine. These data suggest that cell bound IgE is of major importance in the in vitro anaphylactic response and that the direct and reversed in vitro anaphylactic reactions both operate through cell-bound IgE and share a common reaction mechanism.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg T., Johansson S. G. IgE concentrations in children with atopic diseases. A clinical study. Int Arch Allergy Appl Immunol. 1969;36(3):219–232. doi: 10.1159/000230745. [DOI] [PubMed] [Google Scholar]
- GREEN H., FLEISCHER R. A., BARROW P., GOLDBERG B. The cytotoxic action of immune gamma globulin and complement on Krebs ascites tumor cells. II. Chemical studies. J Exp Med. 1959 May 1;109(5):511–521. doi: 10.1084/jem.109.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY J. H., AUSTEN K. F., RAPP H. J. In vitro studies of reversed anaphylaxis with rat cells. Immunology. 1963 May;6:226–245. [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Identification of gamma-E-antibodies as a carrier of reaginic activity. J Immunol. 1967 Dec;99(6):1187–1198. [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Immune mechanisms of reversed type reaginic hypersensitivity. J Immunol. 1969 Sep;103(3):588–595. [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Reversed type allergic skin reactions by anti-gamma-E-globulin antibodies in humans and monkeys. J Immunol. 1968 Mar;100(3):554–562. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Johansson S. G., Bennich H. Histamine release from human leukocytes by anti-gamma E antibodies. J Immunol. 1969 Apr;102(4):884–892. [PubMed] [Google Scholar]
- Johansson S. G. Raised levels of a new immunoglobulin class (IgND) in asthma. Lancet. 1967 Nov 4;2(7523):951–953. doi: 10.1016/s0140-6736(67)90792-1. [DOI] [PubMed] [Google Scholar]
- KELLER R. Effect of antibodies against rat gamma-globulin or rat albumin on isolated peritoneal rat mast cells. Nature. 1962 Jan 20;193:282–283. doi: 10.1038/193282a0. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. A., Carlton J. A. Influence of temperature on the inhibition by colchicine of allergic histamine release. Proc Soc Exp Biol Med. 1969 Apr;130(4):1333–1336. doi: 10.3181/00379727-130-33786. [DOI] [PubMed] [Google Scholar]
- Levy D. A., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XIV. Passive sensitization in vitro of human leukocytes to ragweed pollen antigen. J Immunol. 1966 Aug;97(2):203–212. [PubMed] [Google Scholar]
- Levy D. A., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XV. Enhancement of passive sensitization of human leukocytes by heparin. J Immunol. 1967 Dec;99(6):1062–1067. [PubMed] [Google Scholar]
- Levy D. A., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XVI. In vitro assays of reaginic activity in human sera: effect of therapeutic immunization on seasonal titer changes. J Immunol. 1967 Dec;99(6):1068–1077. [PubMed] [Google Scholar]
- Lichtenstein L. M., Margolis S. Histamine release in vitro: inhibition by catecholamines and methylxanthines. Science. 1968 Aug 30;161(3844):902–903. doi: 10.1126/science.161.3844.902. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Osler A. G. Comparative studies of histamine release and potassium efflux from human leukocytes. Proc Soc Exp Biol Med. 1966 Mar;121(3):808–812. doi: 10.3181/00379727-121-30894. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. S. Radioactive single radial diffusion: a method for increasing the sensitivity of immunochemical quantification of proteins in agar gel. Bull World Health Organ. 1969 Apr;40(4):613–616. [PMC free article] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Sadan N., Rhyne M. B., Mellits E. D., Goldstein E. O., Levy D. A., Lichtenstein L. M. Immunotherapy of pollinosis in children: investigation of the immunologic basis of clinical improvement. N Engl J Med. 1969 Mar 20;280(12):623–627. doi: 10.1056/NEJM196903202801201. [DOI] [PubMed] [Google Scholar]


