Abstract
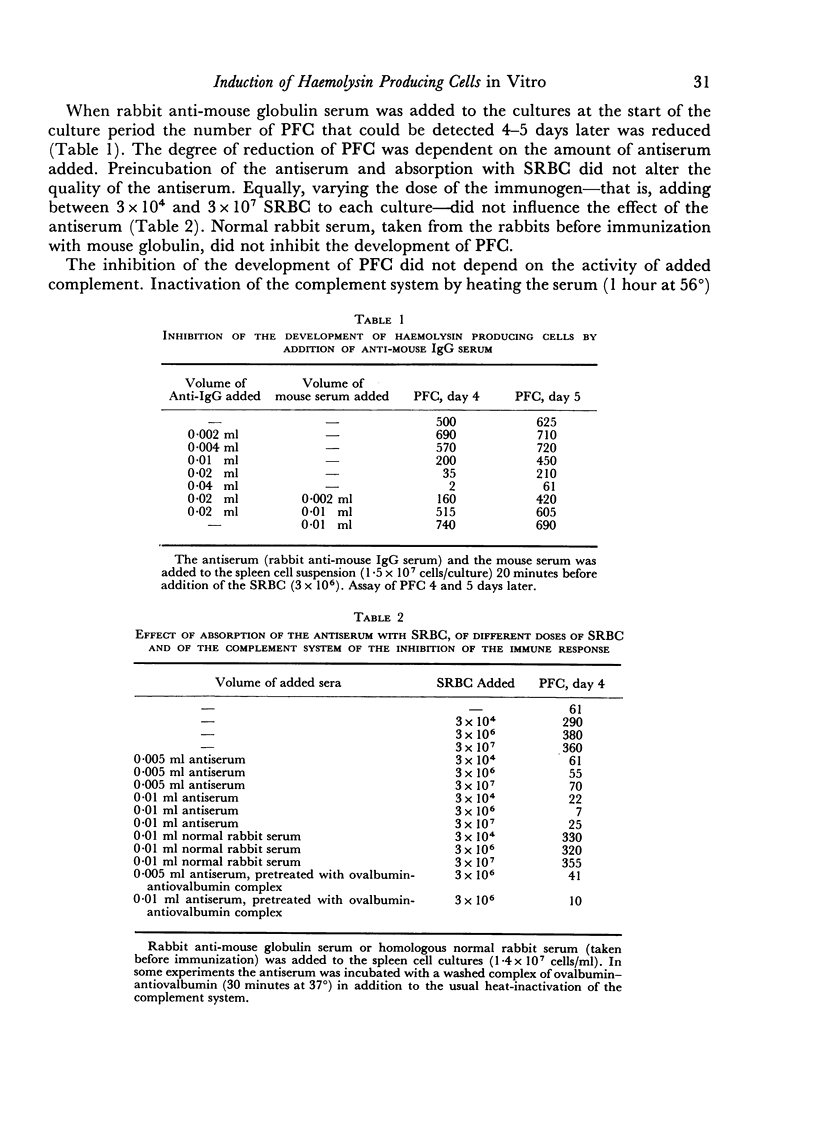
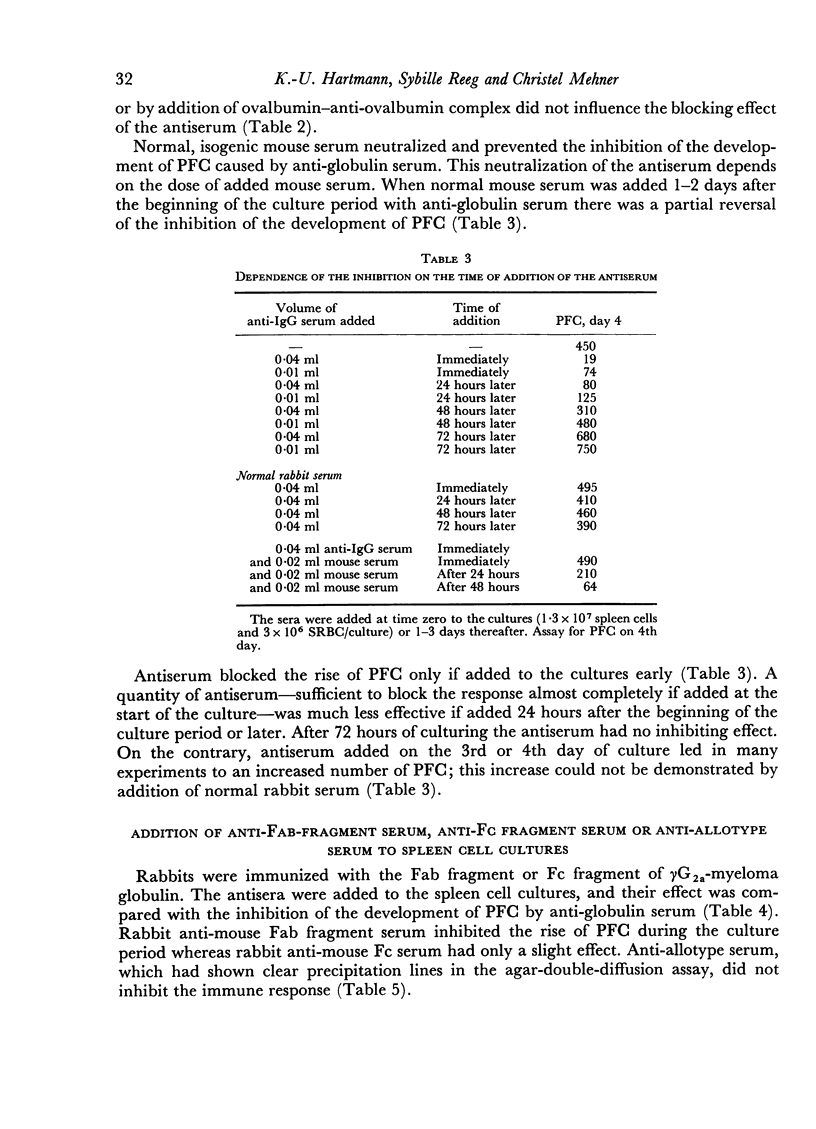
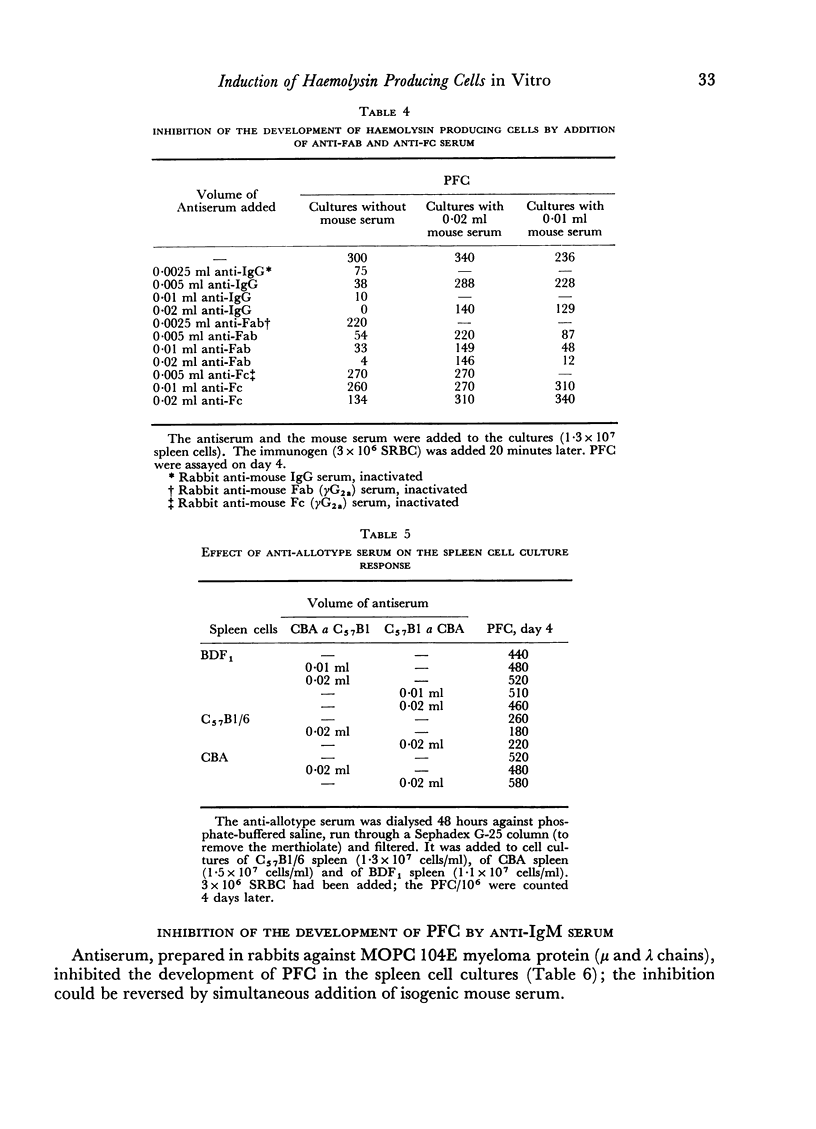
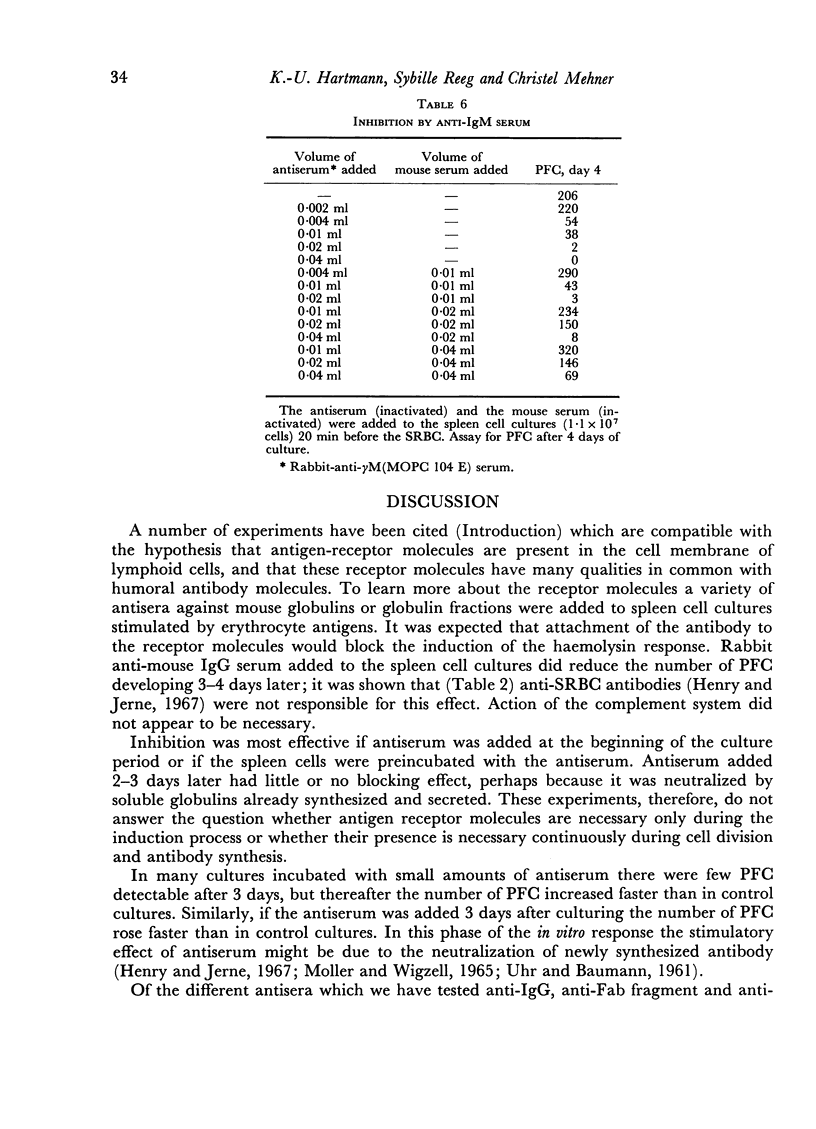
The primary immune response (PFC) of mouse spleen cell cultures to sheep red cells was inhibited by anti-IgG, anti-Fab and anti-IgM, but not by anti-Fc or anti-allotype sera. The inhibition of PFC was independent of complement and could be prevented by addition of normal mouse serum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Byrt P. Specific inactivation of antigen-reactive cells with 125I-labelled antigen. Nature. 1969 Jun 28;222(5200):1291–1292. doi: 10.1038/2221291a0. [DOI] [PubMed] [Google Scholar]
- Bretscher P. A., Cohn M. Minimal model for the mechanism of antibody induction and paralysis by antigen. Nature. 1968 Nov 2;220(5166):444–448. doi: 10.1038/220444a0. [DOI] [PubMed] [Google Scholar]
- DUTTON R. W., EADY J. D. AN IN VITRO SYSTEM FOR THE STUDY OF THE MECHANISM OF ANTIGENIC STIMULATION IN THE SECONDARY RESPONSE. Immunology. 1964 Jan;7:40–53. [PMC free article] [PubMed] [Google Scholar]
- Fuji H., Jerne N. K. Primary immune response in vitro: reversible suppression by anti-globulin antibodies. Ann Inst Pasteur (Paris) 1969 Dec;117(6):801–805. [PubMed] [Google Scholar]
- Greaves M. F., Torrigiani G., Roitt I. M. Blocking of the lymphocyte receptor site for cell mediated hypersensitivity and transplantation reactions by anti-light chain sera. Nature. 1969 May 31;222(5196):885–886. doi: 10.1038/222885a0. [DOI] [PubMed] [Google Scholar]
- Lesley J., Dutton R. W. Antigen receptor molecules: inhibition by antiserum against kappa light chains. Science. 1970 Jul 31;169(3944):487–488. doi: 10.1126/science.169.3944.487. [DOI] [PubMed] [Google Scholar]
- MOLLER G., WIGZELL H. ANTIBODY SYNTHESIS AT THE CELLULAR LEVEL. ANTIBODY-INDUCED SUPPRESSION OF 19S AND 7S ANTIBODY RESPONSE. J Exp Med. 1965 Jun 1;121:969–989. doi: 10.1084/jem.121.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Rogentine G. N., Terry W. D. The transformation of human lymphocytes by monkey antisera to human immunoglobulins. Immunology. 1969 Jan;16(1):123–138. [PMC free article] [PubMed] [Google Scholar]
- SELL S., GELL P. G. STUDIES ON RABBIT LYMPHOCYTES IN VITRO. I. STIMULATION OF BLAST TRANSFORMATION WITH AN ANTIALLOTYPE SERUM. J Exp Med. 1965 Aug 1;122:423–440. doi: 10.1084/jem.122.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S., Globerson A., Feldman M., Haimovich J., Givol D. Specific blocking in vitro of antibody synthesis by affinity labelling reagents. Nature. 1969 Sep 27;223(5213):1374–1375. doi: 10.1038/2231374a0. [DOI] [PubMed] [Google Scholar]
- Sell S. Studies on rabbit lymphocytes in vitro. VI. The induction of blast transformation with sheep antisera to rabbit IgA and IgM. J Exp Med. 1967 Mar 1;125(3):393–400. doi: 10.1084/jem.125.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Ivanyi J. Lymphocyte transformation by H-chain specific anti-immunoglobulin sera. Nature. 1969 Feb 15;221(5181):681–682. doi: 10.1038/221681a0. [DOI] [PubMed] [Google Scholar]
- UHR J. W., BAUMANN J. B. Antibody formation. I. The suppression of antibody formation by passively administered antibody. J Exp Med. 1961 May 1;113:935–957. doi: 10.1084/jem.113.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Andersson B. Cell separation on antigen-coated columns. Elimination of high rate antibody-forming cells and immunological memory cells. J Exp Med. 1969 Jan 1;129(1):23–36. doi: 10.1084/jem.129.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]


