Abstract
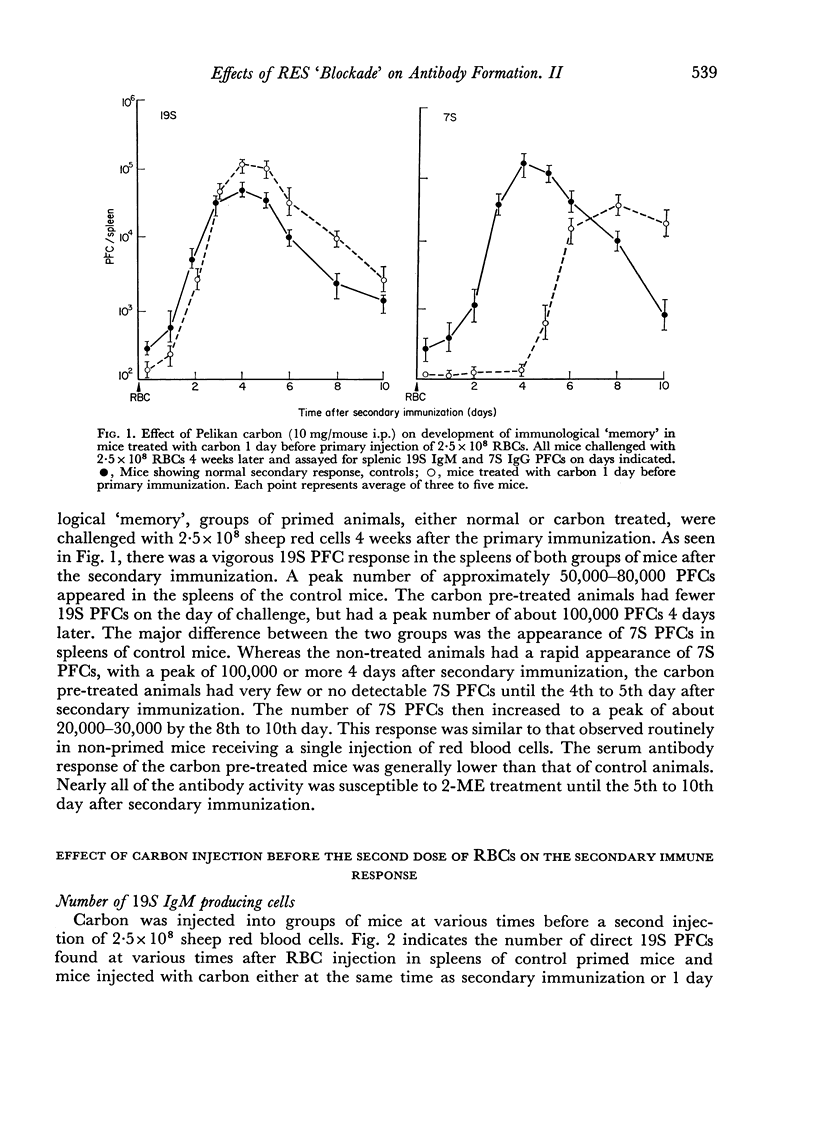
Suppression of the primary immune response by treatment of mice with carbon 1 day before initial immunization markedly interfered with development of immunological `memory', since such mice responded to a subsequent challenge injection of RBCs by formation of mainly IgM PFCs and serum antibody. Appearance of IgG PFCs and 2-ME resistant antibody was delayed several days in these carbon treated animals, indicating failure of a typical secondary response. The immune response of these animals was similar to that of a primary response of control animals to a single injection of red cells.
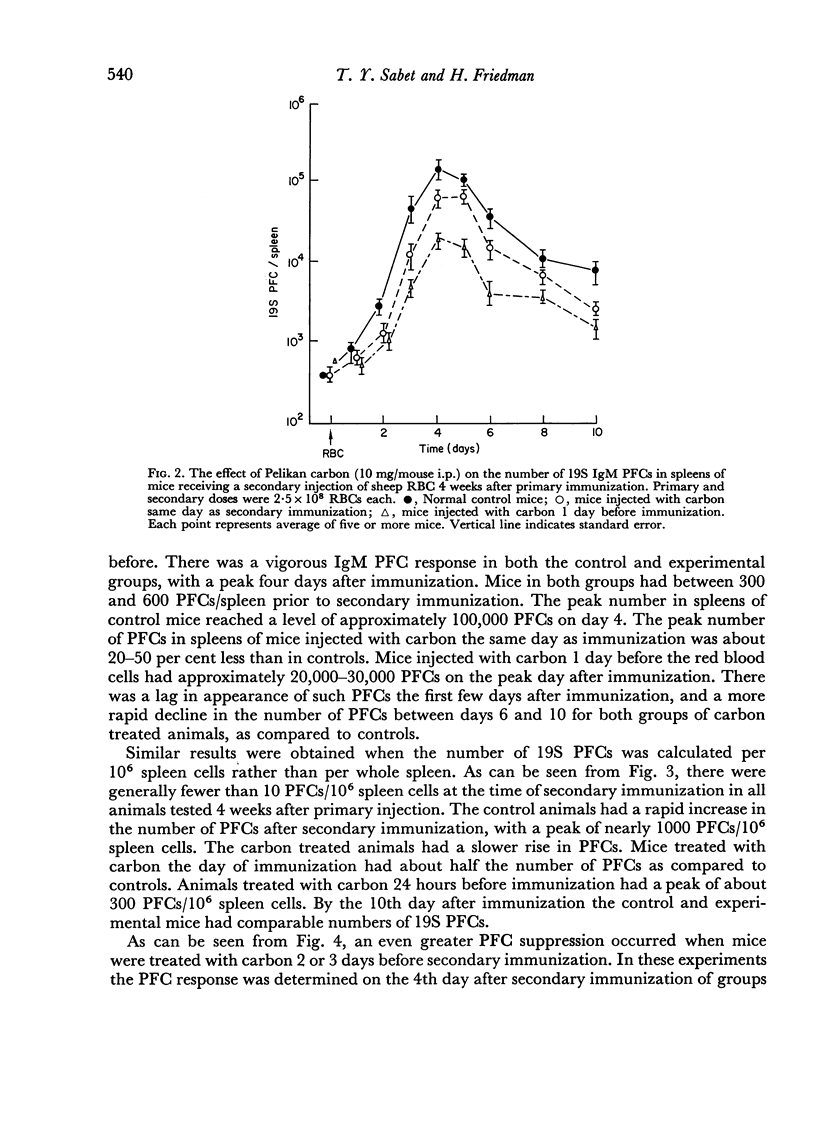
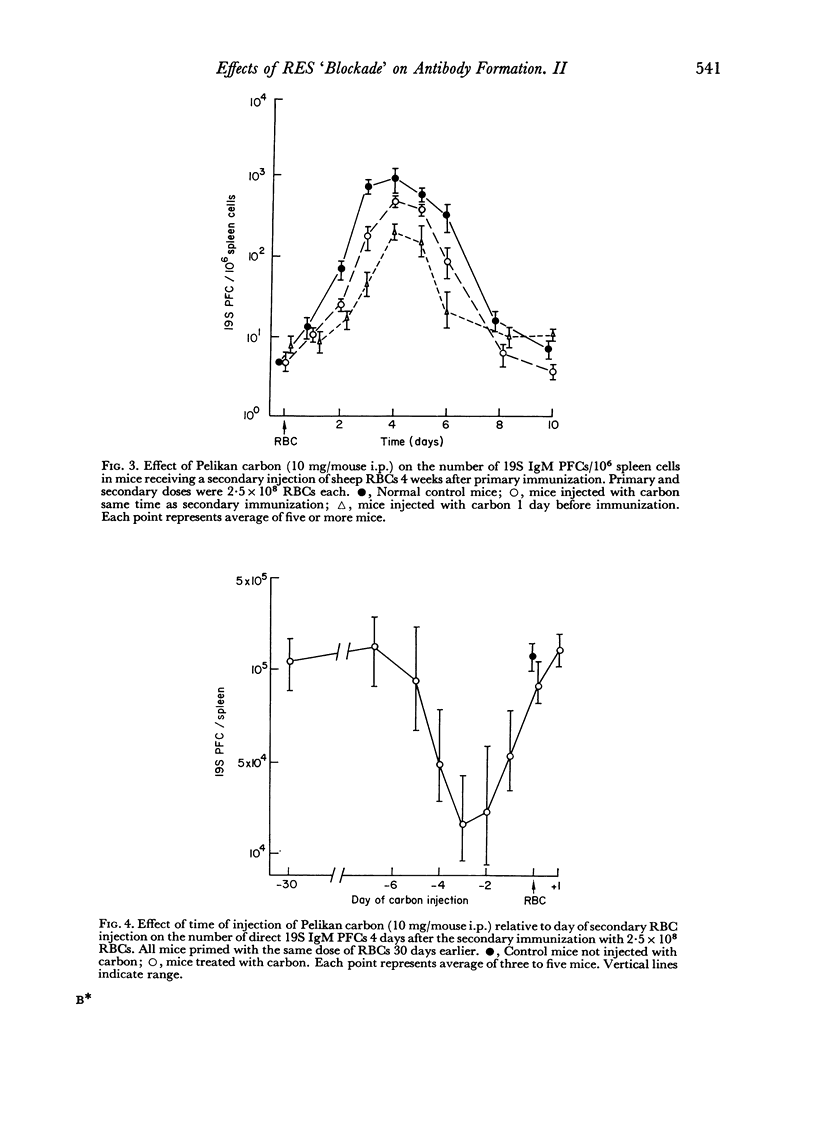
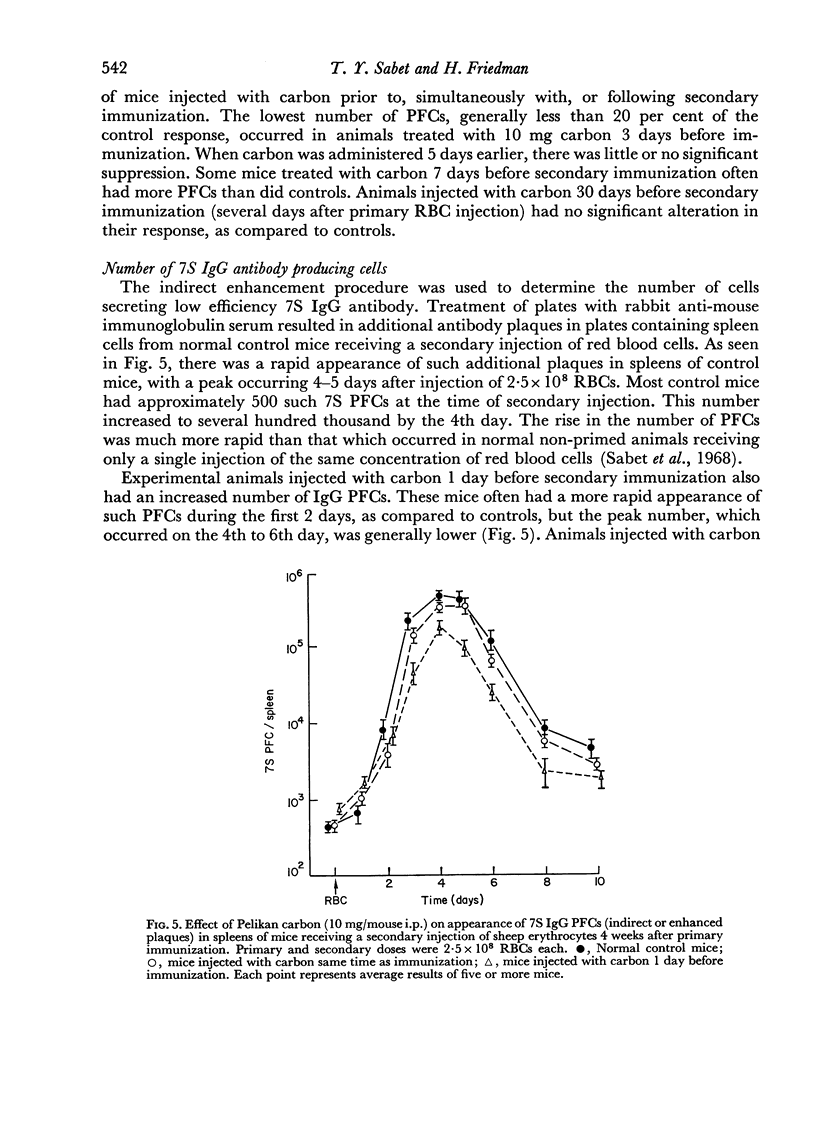
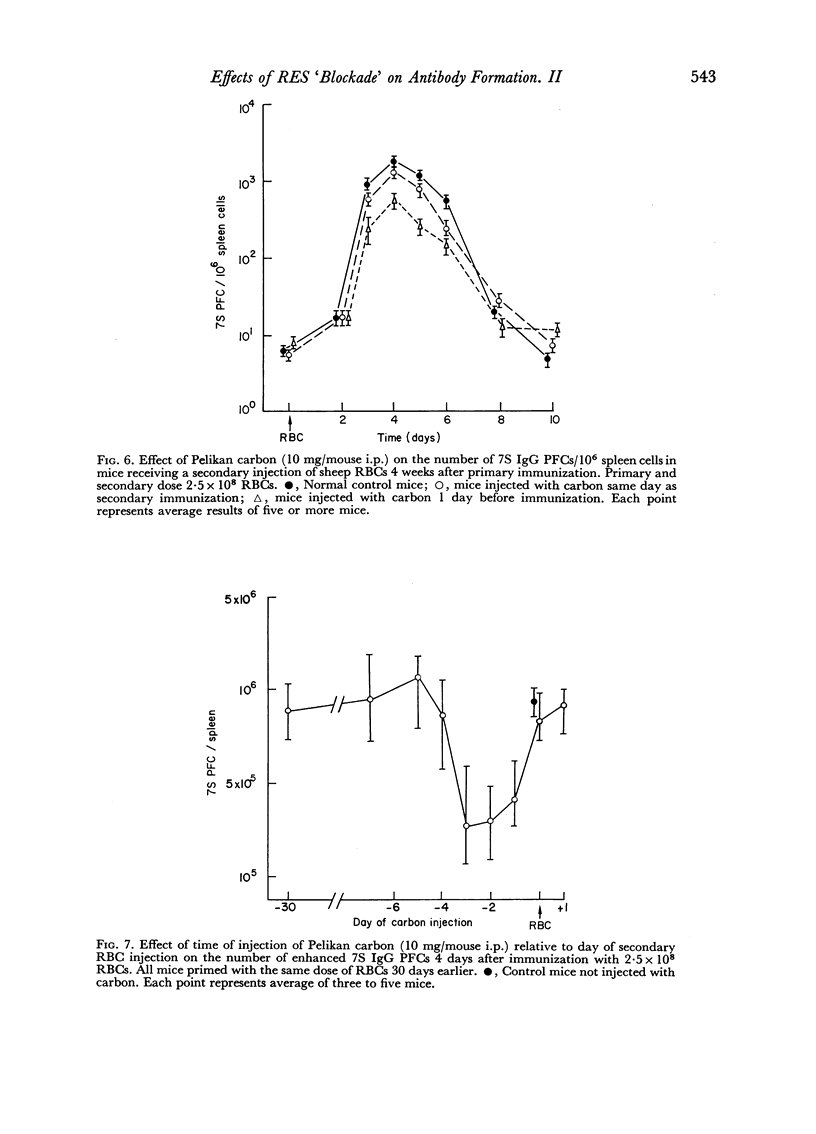
Reticulo-endothelial cell blockade with colloidal carbon suspensions interfered with development of a normal secondary type immune response to sheep red blood cells, as assayed on both the cellular and humoral levels. Fewer antibody PFCs, mainly 19S IgM but also 7S IgG, appeared in spleens of antigen primed mice treated with carbon 1–3 days prior to a challenge injection of red cells, as compared to control primed mice injected with erythrocytes alone. However, the peak day of antibody response was the same for both control and carbon treated animals.
Mice treated with carbon 1–3 days before secondary immunization had much lower peak serum titres, mostly susceptible to 2-ME inactivation.
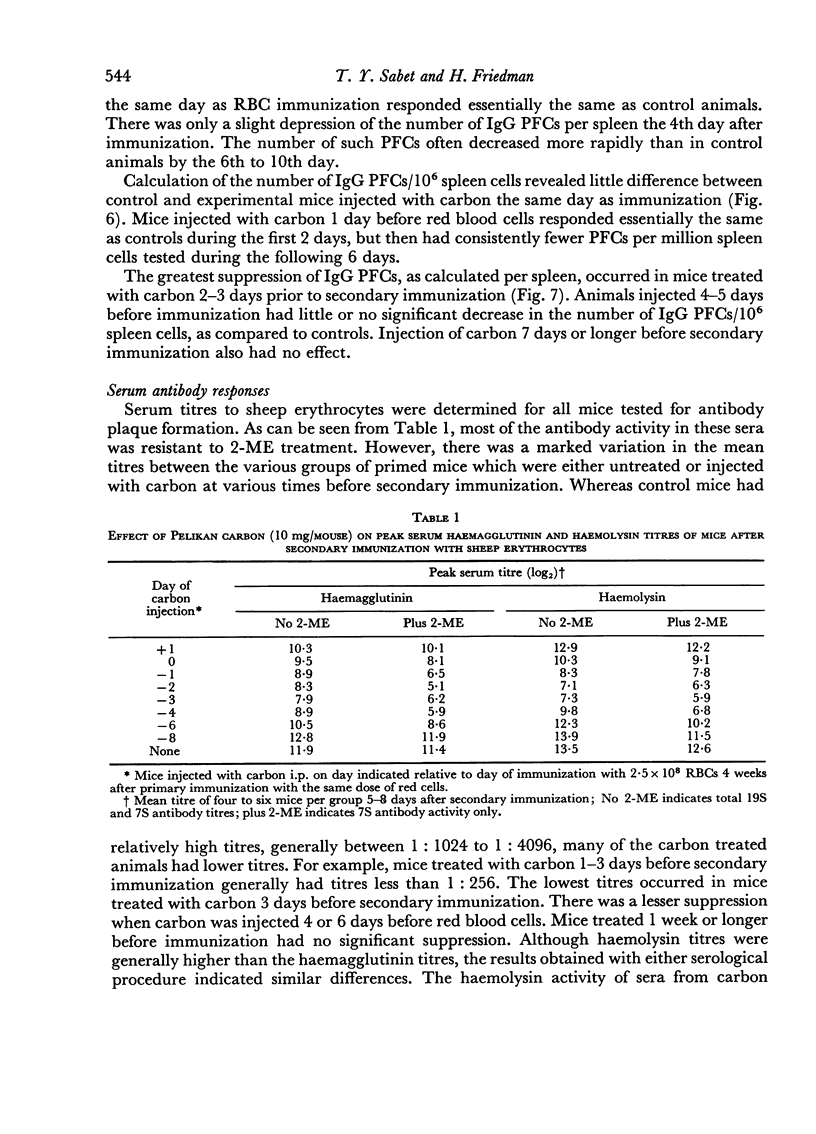
The time of inoculation of carbon in relation to immunization was important since carbon treatment 1–3 days before secondary RBC immunization resulted in maximum suppression. Injection of carbon 5–7 days before resulted in only a slight effect, whereas injection 30 days before had no detectable effect. Injection of carbon simultaneously or after RBC injection had little effect.
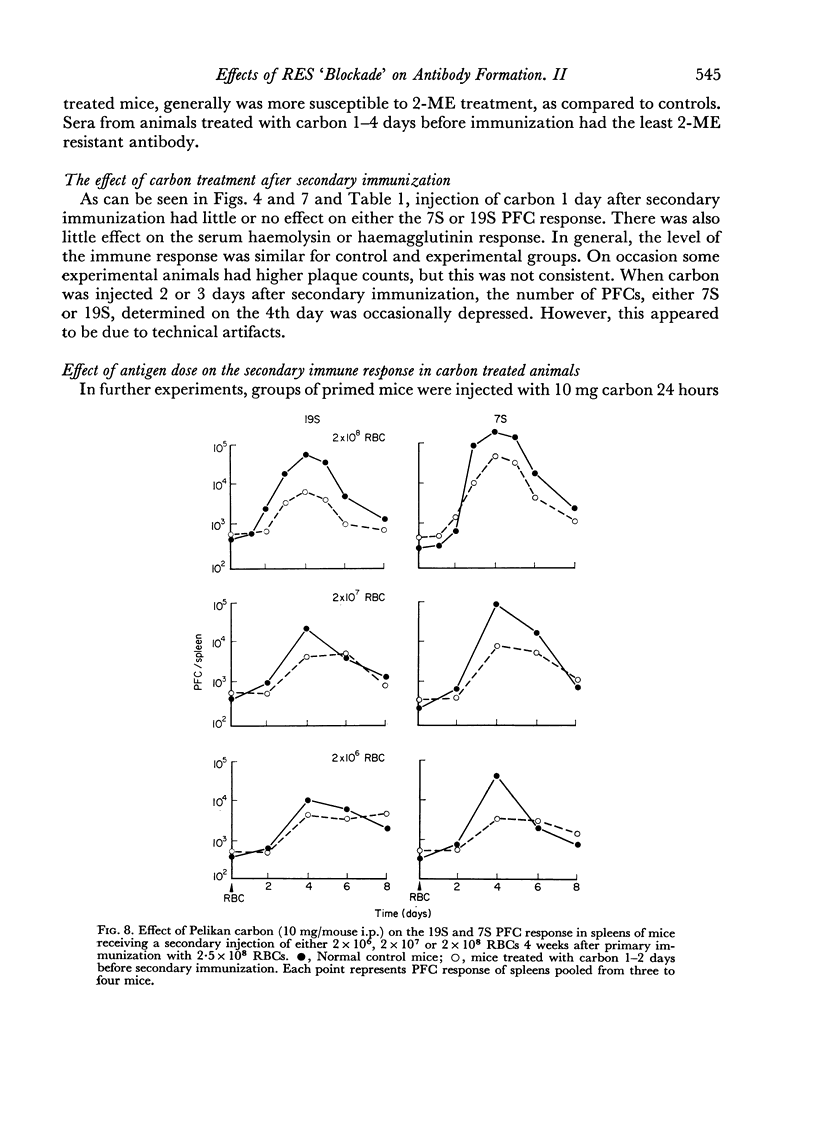
The dose of carbon used for immunosuppression, as well as the concentration of sheep erythrocytes used for immunization affected the number of antibody PFCs and the serum titres in control as well as carbon treated animals.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., RHODES J. M. IMMUNOGENICITY OF ANTIGEN-CONTAINING RIBONUCLEIC ACID PREPARATIONS FROM MACROPHAGES. Nature. 1965 Jan 30;205:470–474. doi: 10.1038/205470a0. [DOI] [PubMed] [Google Scholar]
- Argyris B. F. Role of macrophages in antibody production. Immune response to sheep red blood cells. J Immunol. 1967 Oct;99(4):744–750. [PubMed] [Google Scholar]
- BERENBAUM M. C. EFFECTS OF CARCINOGENS ON IMMUNE PROCESSES. Br Med Bull. 1964 May;20:159–164. doi: 10.1093/oxfordjournals.bmb.a070311. [DOI] [PubMed] [Google Scholar]
- Cruchaud A. The effect of reticuloendothelial blockade on antibody formation and immunologic tolerance. Lab Invest. 1968 Jul;19(1):15–24. [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- Drutz D. J., Koenig M. G., Rogers D. E. Further observations on the mechanism of reticuloendothelial blockade. J Exp Med. 1967 Dec 1;126(6):1087–1098. doi: 10.1084/jem.126.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHMAN M., ADLER F. L. Antibody formation initiated in vitro. II. Antibody synthesis in x-irradiated recipients of diffusion chambers containing nucleic acid derived from macrophages incubated with antigen. J Exp Med. 1963 Apr 1;117:595–602. doi: 10.1084/jem.117.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREI P. C., BENACERRAF B., THORBECKE G. J. PHAGOCYTOSIS OF THE ANTIGEN, A CRUCIAL STEP IN THE INDUCTION OF THE PRIMARY RESPONSE. Proc Natl Acad Sci U S A. 1965 Jan;53:20–23. doi: 10.1073/pnas.53.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN H. DISTRIBUTION OF ANTIBODY PLAQUE FORMING CELLS IN VARIOUS TISSUES OF SEVERAL STRAINS OF MICE INJECTED WITH SHEEP ERYTHROCYTES. Proc Soc Exp Biol Med. 1964 Nov;117:526–530. doi: 10.3181/00379727-117-29628. [DOI] [PubMed] [Google Scholar]
- Fisher S. Stimulation of splenic antigen uptake and of antibody response in mice by India ink or other "blockading" agents. Immunology. 1966 Aug;11(2):127–136. [PMC free article] [PubMed] [Google Scholar]
- Friedman H. P., Stavitsky A. B., Solomon J. M. Induction in vitro of antibodies to phage T2: antigens in the RNA extract employed. Science. 1965 Sep 3;149(3688):1106–1107. doi: 10.1126/science.149.3688.1106. [DOI] [PubMed] [Google Scholar]
- Gallily R., Feldman M. The role of macrophages in the induction of antibody in x-irradiated animals. Immunology. 1967 Feb;12(2):197–206. [PMC free article] [PubMed] [Google Scholar]
- Jenkin C. R., Auzins I., Reade P. C. The synthesis of macroglobulin antibody to a bacterial antigen in mice after treatment with thorotrast. Aust J Exp Biol Med Sci. 1965 Oct;43(5):607–624. doi: 10.1038/icb.1965.46. [DOI] [PubMed] [Google Scholar]
- KOENIG M. G., HEYSSEL R. M., MELLY M. A., ROGERS D. E. THE DYNAMICS OF RETICULOENDOTHELIAL BLOCKADE. J Exp Med. 1965 Jul 1;122:117–142. doi: 10.1084/jem.122.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS L. A. Effect of repeated injections of thorotrast on antibody production. Am J Physiol. 1954 Nov;179(2):285–286. doi: 10.1152/ajplegacy.1954.179.2.285. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Mechanisms of antibody production. Annu Rev Med. 1967;18:81–96. doi: 10.1146/annurev.me.18.020167.000501. [DOI] [PubMed] [Google Scholar]
- PERKINS E. H., MAKINODAN T. THE SUPPRESSIVE ROLE OF MOUSE PERITONEAL PHAGOCYTES IN AGGLUTININ RESPONSE. J Immunol. 1965 May;94:765–777. [PubMed] [Google Scholar]
- Plotz P. H., Talal N., Asofsky R. Assignment of direct and facilitated hemolytic plaques in mice to specific immunoglobulin classes. J Immunol. 1968 Apr;100(4):744–751. [PubMed] [Google Scholar]
- SCHWARTZ R. S., DAMESHEK W. THE ROLE OF ANTIGEN DOSAGE IN DRUG-INDUCED IMMUNOLOGIC TOLERANCE. J Immunol. 1963 May;90:703–710. [PubMed] [Google Scholar]
- STERN K., SPENCER K., FARQUHAR M. Effect of polyvinyl pyrrolidone on immune response in mice. Proc Soc Exp Biol Med. 1955 May;89(1):126–131. doi: 10.3181/00379727-89-21735. [DOI] [PubMed] [Google Scholar]
- Sabet T., Newlin C., Friedman H. Effects of RES "blockade" on antibody-formation. I. Suppressed cellular and humoral haemolysin responses in mice injected with carbon particles. Immunology. 1969 Apr;16(4):433–446. [PMC free article] [PubMed] [Google Scholar]
- Sabet T., Newlin C., Friedman H. The effect of RES blockade on cellular antibody formation to sheep erythrocytes. Proc Soc Exp Biol Med. 1968 May;128(1):274–278. doi: 10.3181/00379727-128-32996. [DOI] [PubMed] [Google Scholar]
- Sterzl J., Ríha I. Detection of cells producing 7S antibodies by the plaque technique. Nature. 1965 Nov 27;208(5013):858–859. doi: 10.1038/208858a0. [DOI] [PubMed] [Google Scholar]
- THORBECKE G. J., BENACERRAF B. The reticulo-endothelial system and immunological phenomena. Prog Allergy. 1962;6:559–598. [PubMed] [Google Scholar]
- WOOD W. B., Jr Phagocytosis, with particular reference to encapsulated bacteria. Bacteriol Rev. 1960 Mar;24(1):41–49. doi: 10.1128/br.24.1.41-49.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]


