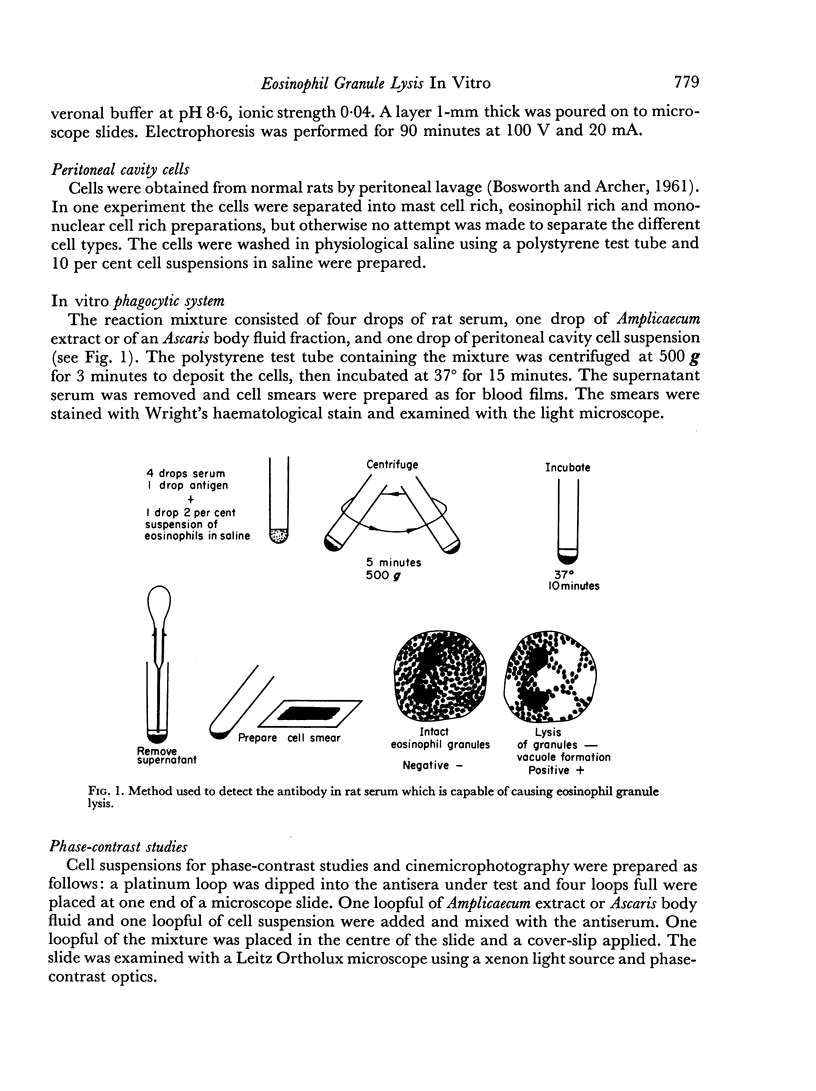
Abstract
A simple test system is described, for the demonstration of antigen—antibody reactions capable of causing eosinophil granule lysis in vitro. The antigen preparations used were extracts of the nematode Amplicaecum robertsi and body fluid of Ascaris suum. Antisera were obtained from rats infested with Amplicaecum. Eosinophils were obtained from the peritoneal cavity of normal rats. Centrifugation of the cells to form a cell button was an essential step in the procedure. Lysis of eosinophils occurred with antiserum obtained from the animals between the 12th and 32nd days of infestation with Amplicaecum, and was accompanied by vacuole formation in macrophages and mast cell disruption. The reaction was most pronounced during the 3rd week. Serum from adrenalectomized infested animals caused the most marked changes in eosinophils. Serum from cortisonetreated infested animals failed to cause eosinophil changes.
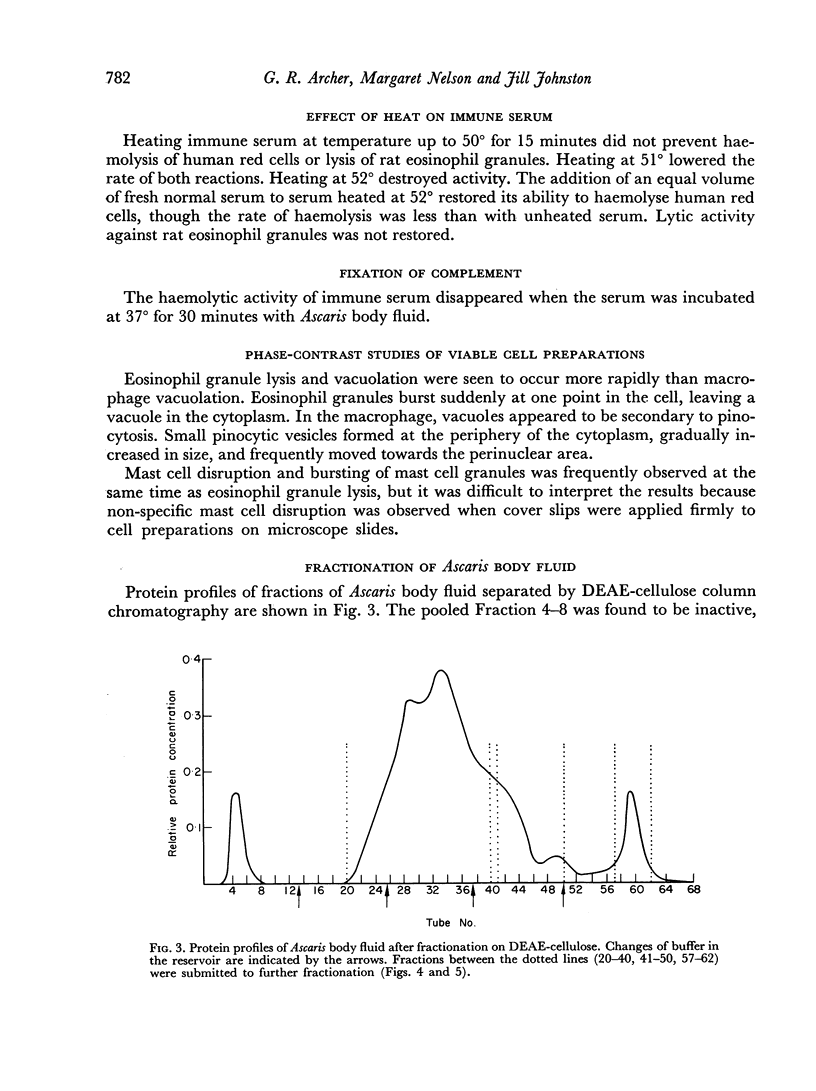
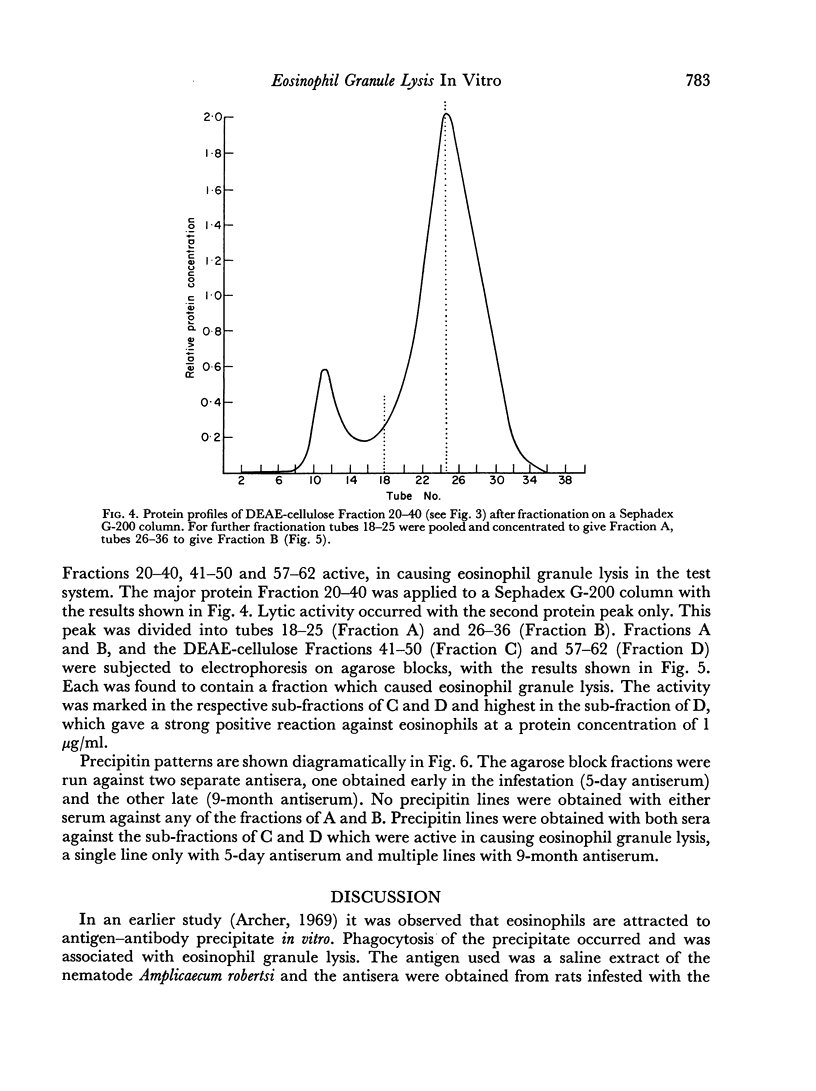
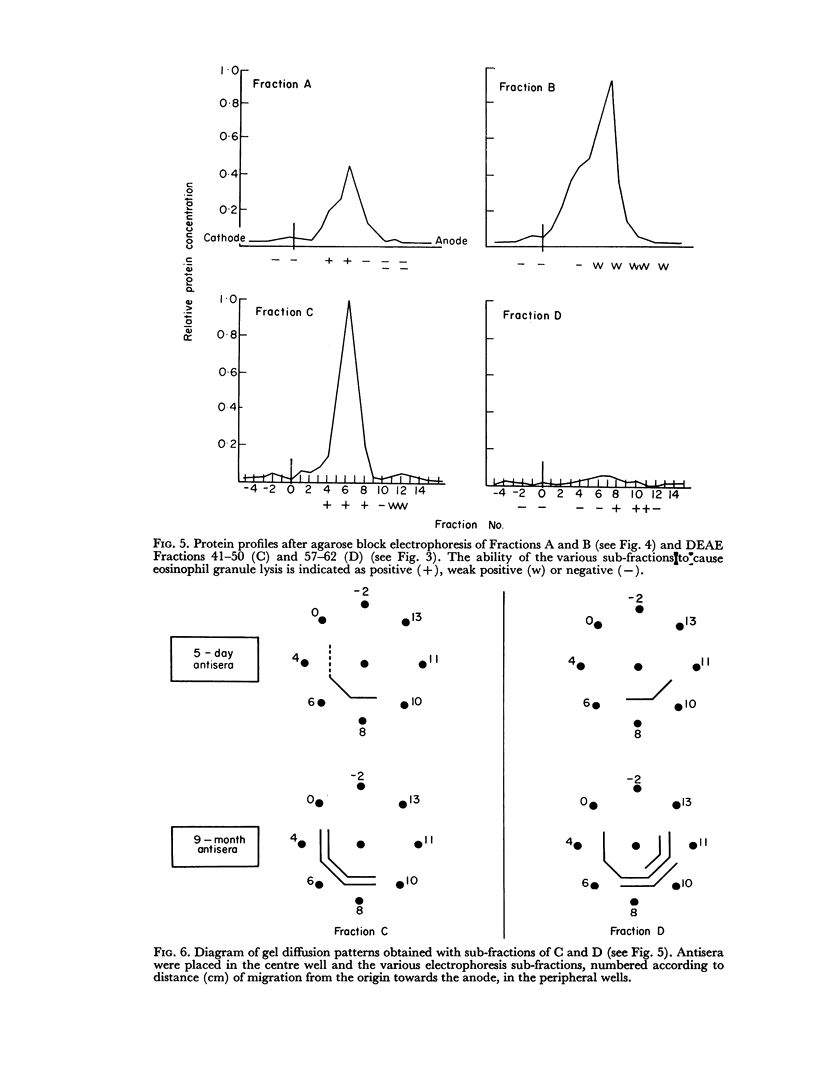
Attempts at purification of the antigen in Ascaris body fluid resulted in two fractions with marked activity in the test system. The same two fractions were found to form precipitin lines on agarose gel diffusion against rat antiserum.
It is postulated that antigen—antibody complexes soluble in low concentration were responsible for the changes observed in the eosinophils, macrophages and mast cells. One or more labile factors in the serum were found to be necessary for eosinophil granule lysis. The evidence, though incomplete, would favour the suggestion that both labile antibody and complement were necessary.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHER G. T., HIRSCH J. G. MOTION PICTURE STUDIES ON DEGRANULATION OF HORSE EOSINOPHILS DURING PHAGOCYTOSIS. J Exp Med. 1963 Aug 1;118:287–294. doi: 10.1084/jem.118.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. T., McGovern V. J. Mast cell changes in rats with eosinophilia. J Pathol Bacteriol. 1968 Jan;95(1):217–224. doi: 10.1002/path.1700950126. [DOI] [PubMed] [Google Scholar]
- Archer G. T. Mechanism of eosinophilia and mast cell disruption in rats infected with the parasite Amplicaecum robertsi. Pathology. 1969 Apr;1(2):133–140. doi: 10.3109/00313026909061047. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Austen K. F. Mechanisms of immunologic injury of rat peritoneal mast cells. I. The effect of phosphonate inhibitors on the homocytotropic antibody-mediated histamine release and the first component of rat complement. J Exp Med. 1966 Sep 1;124(3):379–395. doi: 10.1084/jem.124.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968 Feb 1;127(2):371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Induction of erythema-wheal reactions by soluble antigen-gammaE antibody complexes in humans. J Immunol. 1968 Jul;101(1):68–78. [PubMed] [Google Scholar]
- Johansson S. G., Mellbin T., Vahlquist B. Immunoglobulin levels in Ethiopian preschool children with special reference to high concentrations of immunoglobulin E (IgND). Lancet. 1968 May 25;1(7552):1118–1121. doi: 10.1016/s0140-6736(68)90187-6. [DOI] [PubMed] [Google Scholar]
- Jones V. E., Ogilvie B. M. Reaginic antibodies and immunity to Nippostrongylus brasiliensis in the rat. II. Some properties of the antibodies and antigens. Immunology. 1967 May;12(5):583–597. [PMC free article] [PubMed] [Google Scholar]
- KENT N. H. Seminar on immunity to parasitic helminths. V. Antigens. Exp Parasitol. 1963 Feb;13:45–56. doi: 10.1016/0014-4894(63)90053-5. [DOI] [PubMed] [Google Scholar]
- LITT M. EOSINOPHILS AND ANTIGEN-ANTIBODY REACTIONS. Ann N Y Acad Sci. 1964 Aug 27;116:964–985. doi: 10.1111/j.1749-6632.1964.tb52562.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOTA I. THE MECHANISM OF ANAPHYLAXIS. I. PRODUCTION AND BIOLOGICAL PROPERTIES OF 'MAST CELL SENSITIZING' ANTIBODY. Immunology. 1964 Nov;7:681–699. [PMC free article] [PubMed] [Google Scholar]
- Mota I. Biological characterization of mouse 'early' antibodies. Immunology. 1967 Mar;12(3):343–348. [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- SAMTER M., KOFOED M. A., PIEPER W. A factor in lungs of anaphylactically shocked guinea pigs which can induce eosinophilia in normal animals. Blood. 1953 Dec;8(12):1078–1090. [PubMed] [Google Scholar]