Abstract
The occurrence of immunoglobulin determinants on the surface of lymphocytes from human blood was assessed by indirect immunofluorescent staining of living cells after cultivation with phytohaemagglutinin or other stimulants. While antisera to γ, μ or α-determinants only stained a few cells, antisera to light chain determinants stained a larger proportion of the cells. Positive staining was recognized as `ring' staining comprising smaller or larger parts of the cell surface. The specificity of staining was ascertained by several types of controls.
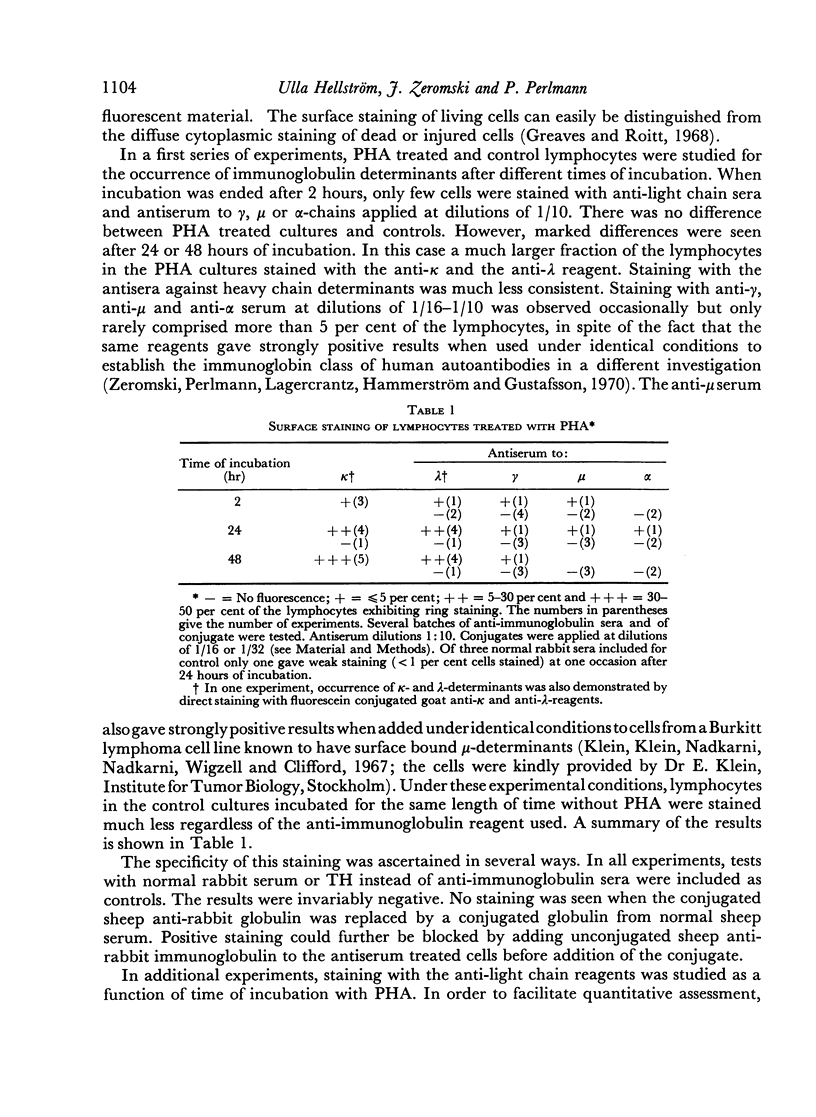
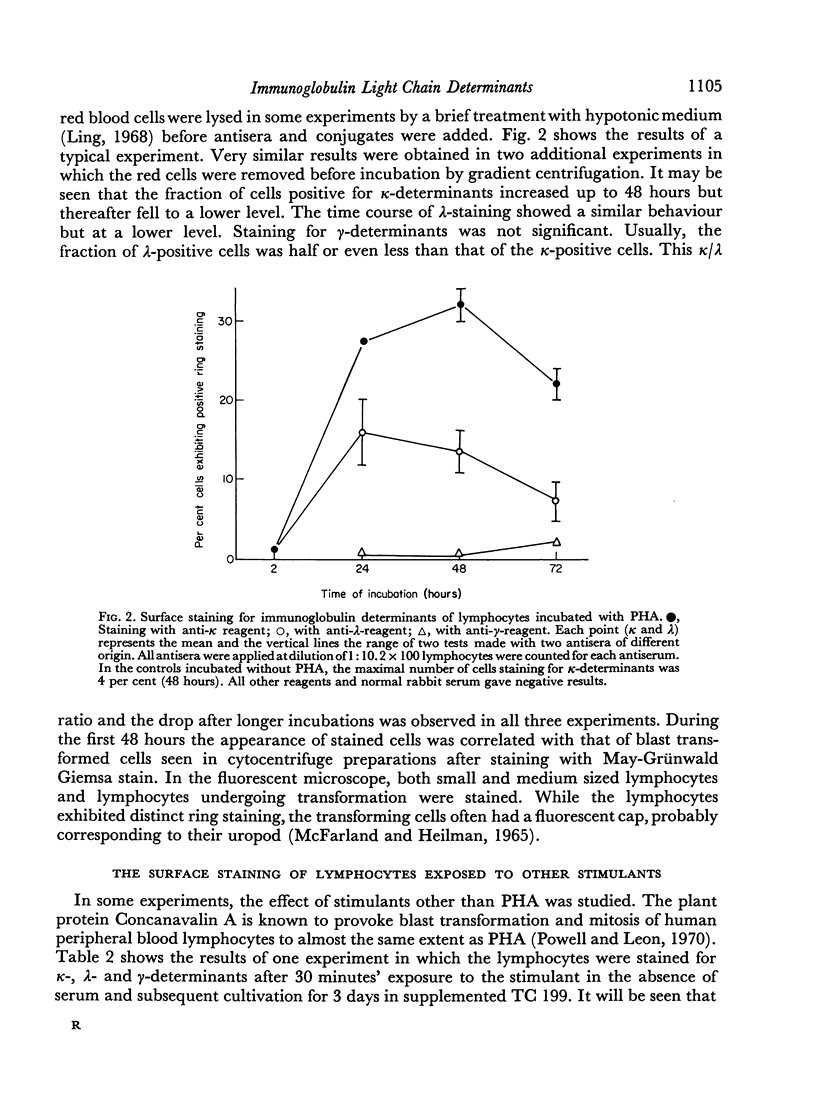
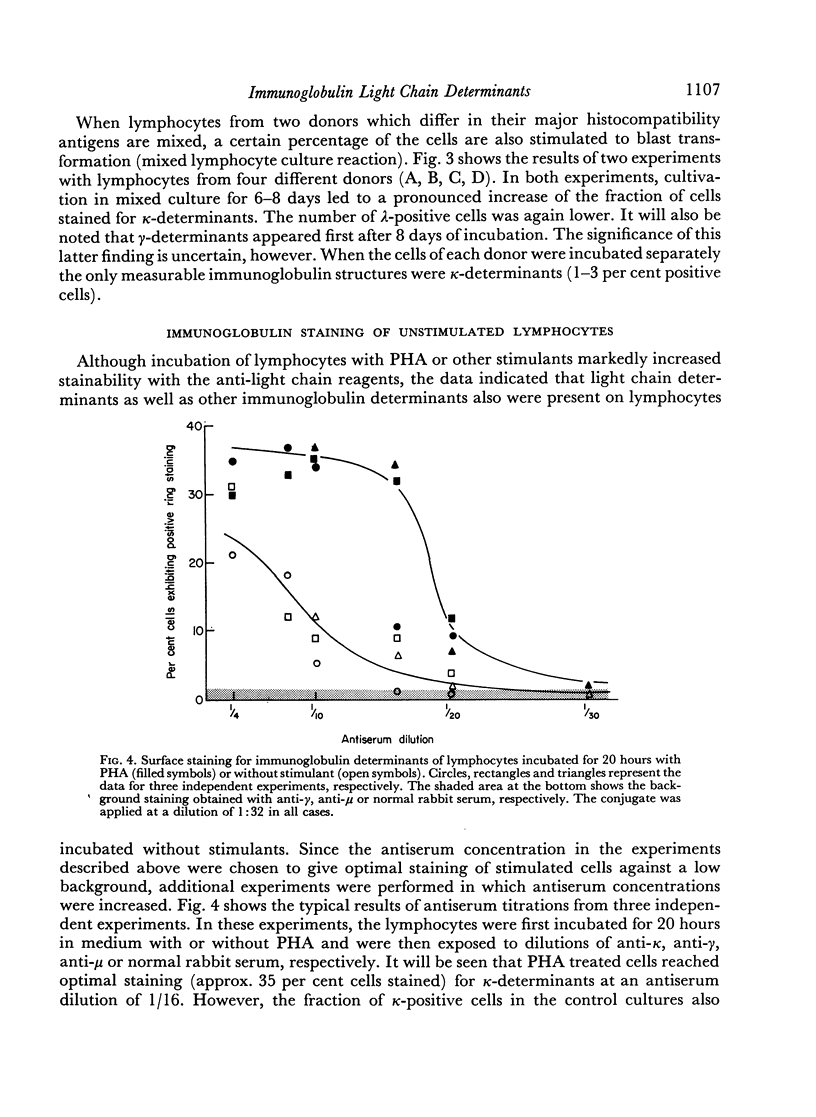
After 48 hours of cultivation, anti-κ serum, applied at dilutions of 1:10–1:16 stained about 35–50 per cent and anti-λ serum about 15–20 per cent of the cells in the PHA cultures but only 3–5 per cent in the cultures incubated without PHA. When the antisera were applied at higher concentrations, positive light chain staining was also seen in the unstimulated cultures. At the highest concentrations, which could be used without increasing the non-specific background, the maximum number of κ-positive cells in the unstimulated cultures was approximately 25 per cent. Antiserum titrations showed that about 5–10 times less antiserum was needed to stain the optimal fraction of PHA treated cells. No increased staining of heavy chain determinants was achieved by increasing antiserum concentrations under the present conditions.
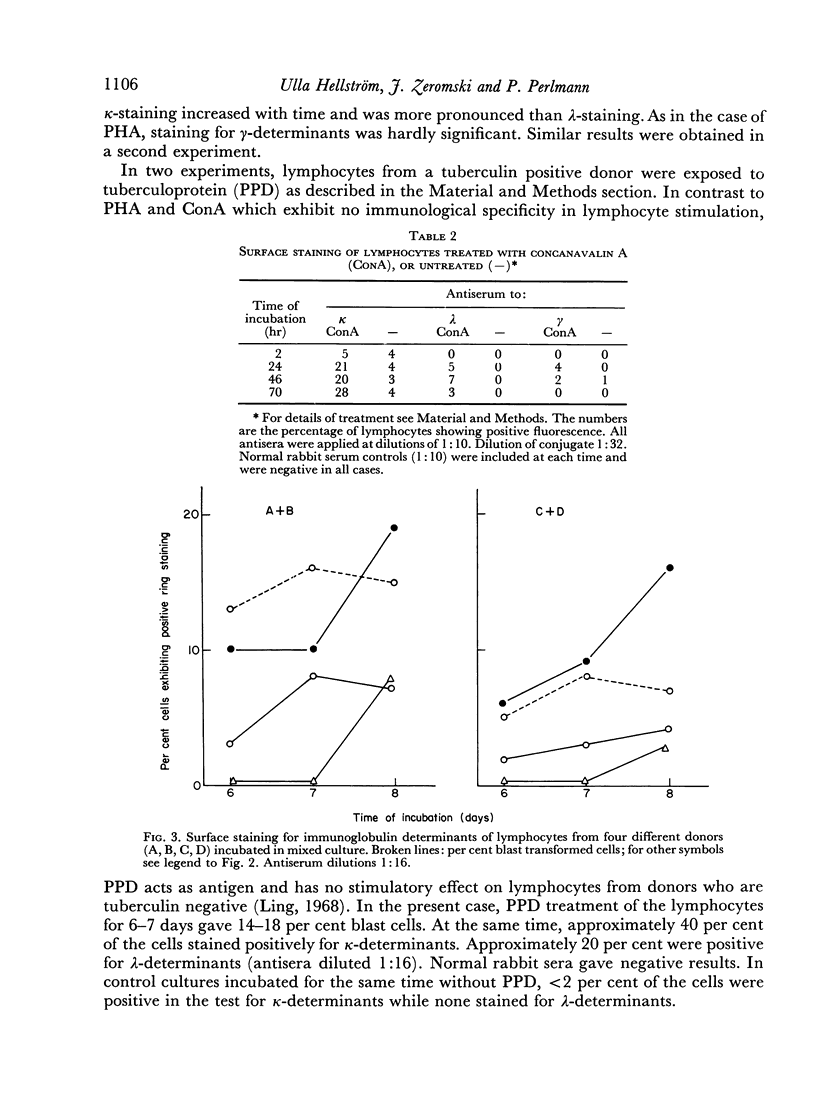
Similar results were obtained with lymphocytes stimulated by 3 days of incubation with concanavalin A, or by 6–7 days of incubation under mixed culture conditions. Lymphocytes of a tuberculin positive donor also gave increased staining for light chain determinants after incubation for 6–7 days with antigen (PPD).
The results indicate that lymphocyte stimulation is accompanied by increased amounts of surface bound immunoglobulins. At the present stage of knowledge, several explanations may account for the fact that light chain determinants are primarily accessible for staining.
The above results were obtained under conditions in which no protein was present in the washings performed during processing for immunofluorescence. In the presence of low concentrations of protein more than 60 per cent of both unstimulated and stimulated cells stained for light chain determinants, while staining for heavy chain determinants remained unchanged and at a low level. It is possible that protein-free washing removed a more loosely adsorbed immunoglobulin fraction passed on from producing to neighbouring non-producing cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bert G., Massaro A. L., Di Cossano D. L., Maja M. Electrophoretic study of immunoglobulins and immunoglobulin sub-units on the surface of human peripheral blood lymphocytes. Immunology. 1969 Jul;17(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Biozzi G., Binaghi R. A., Stiffel C., Mouton D. Production of different classes of immunoglobulins by individual cells in the guinea-pig. Immunology. 1969 Mar;16(3):349–359. [PMC free article] [PubMed] [Google Scholar]
- Byrt P., Ada G. L. An in vitro reaction between labelled flagellin or haemocyanin and lymphocyte-like cells from normal animals. Immunology. 1969 Oct;17(4):503–516. [PMC free article] [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Coombs R. R., Feinstein A., Wilson A. B. Immunoglobulin determinants on the surface of human lymphocytes. Lancet. 1969 Nov 29;2(7631):1157–1160. doi: 10.1016/s0140-6736(69)92484-2. [DOI] [PubMed] [Google Scholar]
- Coombs R. R., Franks D. Immunological reactions involving two cell types. Prog Allergy. 1969;13:174–272. [PubMed] [Google Scholar]
- Coombs R. R., Gurner B. W., Janeway C. A., Jr, Wilson A. B., Gell P. G., Kelus A. S. Immunoglobulin determinants on the lymphocytes of normal rabbits. I. Demonstration by the mixed antiglobulin reaction of determinants recognized by anti-gamma, anti-mu, anti-Fab and anti-allotype sera, anti-As4 and anti-As6. Immunology. 1970 Mar;18(3):417–429. [PMC free article] [PubMed] [Google Scholar]
- Doré C. F., Balfour B. M. A device for preparing cell spreads. Immunology. 1965 Oct;9(4):403–405. [PMC free article] [PubMed] [Google Scholar]
- GREENWALT T. J., GAJEWSKI M., McKENNA J. L. A new method for preparing buffy coat-poor blood. Transfusion. 1962 Jul-Aug;2:221–229. doi: 10.1111/j.1537-2995.1962.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Greaves M. F. Biological effects of anti-immunoglobulins: evidence for immunoglobulin receptors on 'T' and 'B' lymphocytes. Transplant Rev. 1970;5:45–75. doi: 10.1111/j.1600-065x.1970.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Roitt I. M. The effect of phytohaemagglutinin and other lymphocyte mitogens on immunoglobulin synthesis by human peripheral blood lymphocytes in vitro. Clin Exp Immunol. 1968 Jun;3(5):393–412. [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Torrigiani G., Roitt I. M. Blocking of the lymphocyte receptor site for cell mediated hypersensitivity and transplantation reactions by anti-light chain sera. Nature. 1969 May 31;222(5196):885–886. doi: 10.1038/222885a0. [DOI] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surgace IgM specificity on cells derived from a Burkitt's lymphoma. Lancet. 1967 Nov 18;2(7525):1068–1070. doi: 10.1016/s0140-6736(67)90340-6. [DOI] [PubMed] [Google Scholar]
- MCFARLAND W., HEILMAN D. H. LYMPHOCYTE FOOT APPENDAGE: ITS ROLE IN LYMPHOCYTE FUNCTION AND IN IMMUNOLOGICAL REACTIONS. Nature. 1965 Feb 27;205:887–888. doi: 10.1038/205887a0. [DOI] [PubMed] [Google Scholar]
- Mason S., Warner N. L. The immunoglobulin nature of the antigen recognition site on cells mediating transplantation immunity and delayed hypersentivity. J Immunol. 1970 Mar;104(3):762–765. [PubMed] [Google Scholar]
- McConnell I., Munro A., Gurner B. W., Coombs R. R. Studies on actively allergized cells. I. The cyto-dynamics and morphology of rosete-forming lymph node cells in mice and inhibition of rosette-formation with antibody to mouse immunoglobulins. Int Arch Allergy Appl Immunol. 1969;35(3):209–227. [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H. Contactual lysis of antibody-coated chicken erythrocytes by purified lymphocytes. Cell Immunol. 1970 Sep;1(3):300–315. doi: 10.1016/0008-8749(70)90051-1. [DOI] [PubMed] [Google Scholar]
- Powell A. E., Leon M. A. Reversible interaction of human lymphocytes with the mitogen concanavalin A. Exp Cell Res. 1970 Oct;62(2):315–325. doi: 10.1016/0014-4827(70)90560-4. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- SELL S., GELL P. G. STUDIES ON RABBIT LYMPHOCYTES IN VITRO. I. STIMULATION OF BLAST TRANSFORMATION WITH AN ANTIALLOTYPE SERUM. J Exp Med. 1965 Aug 1;122:423–440. doi: 10.1084/jem.122.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. Studies on rabbit lymphocytes in vitro. V. The induction of blast transformation with sheep antisera to rabbit IgG subunits. J Exp Med. 1967 Feb 1;125(2):289–301. doi: 10.1084/jem.125.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulitzeanu D., Naor D. The affinity of radioiodinated BSA for lymphoid cells. II. Binding of 125I-BSA to lymphoid cells of normal mice. Int Arch Allergy Appl Immunol. 1969;35(6):564–578. doi: 10.1159/000230210. [DOI] [PubMed] [Google Scholar]
- Wigzell H., Andersson B. Cell separation on antigen-coated columns. Elimination of high rate antibody-forming cells and immunological memory cells. J Exp Med. 1969 Jan 1;129(1):23–36. doi: 10.1084/jem.129.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeromski J., Perlmann P., Lagercrantz R., Hammarström S., Gustafsson B. E. Immunological studies in ulcerative colitis. VII. Anti-colon antibodies of different immunoglobulin classes. Clin Exp Immunol. 1970 Oct;7(4):469–475. [PMC free article] [PubMed] [Google Scholar]



