Abstract
We analyzed whether sister chromatids are continuously aligned in meristematic and endopolyploid Arabidopsis interphase nuclei by studying sister-chromatid alignment at various chromosomal positions. FISH with individual BACs to flow-sorted 4C root and leaf nuclei frequently yielded more than two hybridization signals, indicating incomplete or absent sister-chromatid alignment. Up to 100% of 8C, 16C, and 32C nuclei showed no sister-chromatid alignment at defined positions. Simultaneous FISH with BACs from different chromosomal positions revealed more frequent sister-chromatid alignment in terminal than in midarm positions. Centromeric positions were mainly aligned up to a ploidy level of 16C but became separated or dispersed in 32C nuclei. DNA hypomethylation (of the whole genome) and transcriptional activity (at FWA gene position) did not impair sister-chromatid alignment. Only 6.1% of 4C leaf nuclei showed sister-chromatid separation of the entire chromosome 1 top arm territories. Homozygous transgenic tandem repeat (lac operator) arrays showing somatic homologous pairing more often than average euchromatic loci did not promote an increased frequency of sister-chromatid alignment. The high frequency of separated sister-chromatid arm positions in ≥4C nuclei suggests that sister-chromatid cohesion is variable, dynamic, and not obligatory along the entire chromosome arm in meristematic and differentiated Arabidopsis nuclei.
THE colinear alignment of sister chromatids is defined as “cohesion” (Maguire 1990; Miyazaki and Orr-Weaver 1994). It is widely assumed that sister chromatids are aligned from replication in S phase until the onset of anaphase to ensure postreplicational recombination repair as well as correct segregation of eukaryotic nuclear genomes from cell to cell and from generation to generation. A ring-shaped complex of cohesin proteins apparently mediates cohesion of newly replicated sister chromatids until complete bipolar orientation is achieved during metaphase (for recent reviews see Biggins and Murray 1999; Hirano 2000; Koshland and Guacci 2000; Campbell and Cohen-Fix 2002; Haering and Nasmyth 2003; Hagstrom and Meyer 2003; Jessberger 2003; Uhlmann 2003, 2004; Nasmyth and Schleiffer 2004; Riedel et al. 2004; Nasmyth 2005).
Distinct mechanisms to align/separate chromatids at specific chromosomal domains such as centromeres, telomeres, and nucleolus organizing regions (NORs) (Rieder and Cole 1999; Warren et al. 2000; D'Amours et al. 2004; Dynek and Smith 2004; Pereira and Schiebel 2004; Sullivan et al. 2004; Tang et al. 2004; Yalon et al. 2004; Watanabe 2005) seem to be related to the different functions of these regions. Furthermore, cohesins are involved in chromosome condensation (with condensins), regulation of gene expression, postreplicational recombination repair, and meiotic recombination (van Heemst and Heyting 2000; Lee and Orr-Weaver 2001; Hagstrom and Meyer 2003; Jessberger 2003; Morrison et al. 2003; Glynn et al. 2004; Lengronne et al. 2004; Revenkova et al. 2004; Webber et al. 2004). For repair of double-strand breaks in G2 nuclei a de novo recruitment of cohesins to break positions seems to be required (Kim et al. 2002; Ünal et al. 2004; Ström et al. 2004).
The knowledge on sister-chromatid cohesion is based mainly on investigations in yeast, Sordaria, Drosophila, Caenorhabditis, Xenopus, chicken, mice, and humans. Cohesin genes in plants acting during mitosis and meiosis were reported for Arabidopsis (Dong et al. 2001; Mercier et al. 2001, 2003; Liu et al. 2002; Cai et al. 2003; Lam et al. 2005).
Cohesins are not randomly distributed along chromosomes but rather located at specific loci. In yeast, these loci are represented mainly by intergenic A + T-rich regions and also by telomeric and centromeric regions. The average extension of cohesion sites is 0.8–1.0 kb (Blat and Kleckner 1999; Megee et al. 1999; Tanaka et al. 1999; Laloraya et al. 2000) separated by ∼11-kb intervals. Activation of transcription mediates repositioning of cohesins (Glynn et al. 2004; Lengronne et al. 2004). Due to the close spacing of cohesion sites, fluorescence in situ hybridization (FISH) signals from sister chromatids cannot be distinguished in yeast (Guacci et al. 1994; Blat and Kleckner 1999). However, in cultured human fibroblasts in G2, sister chromatids may occupy considerably distant positions when probed for distinct loci by FISH (Volpi et al. 2001). In Drosophila, in spite of somatic pairing of homologs, chromatin tagging with a recombinant GFP-Lac repressor protein revealed regular sister-chromatid separation at the tagged locus during mid-G2 before first male meiosis (Vazquez et al. 2002). Applying the same chromatin tagging system to Arabidopsis, a higher frequency of homologous pairing was found in somatic 2C nuclei for the transgenic tandem repeats (lac operator arrays and also HPT1 repeats) than for average euchromatin regions (Pecinka et al. 2005), but sister-chromatid alignment in 4C nuclei was not investigated. The greater range of movement of GFP-tagged lac operator loci in endopolyploid than in 2C Arabidopsis nuclei (Kato and Lam 2003) could be considered an indirect indication of incomplete sister-chromatid alignment in endopolyploid nuclei.
During preliminary investigations we occasionally found three or four instead of one or two FISH signals for chromosome-specific ∼100-kb segments in 4C nuclei of Arabidopsis thaliana, indicating that not only homologs but also sister chromatids may occupy separate positions within a nucleus. Therefore, we became motivated to study the degree of sister-chromatid alignment (close spatial vicinity of identical segments) in A. thaliana nuclei of meristematic and differentiated tissues. For this purpose we used fluorescence in situ hybridization of DNA sequences from different positions along chromosomes. A. thaliana is an endopolyploid species; i.e., differentiated cells may undergo endoreplication cycles without nuclear division between replication phases. The 4C nuclei resulting from the first replication step theoretically could correspond to the mitotic G2 stage. Therefore, we tested meristematic and differentiated 4C nuclei as to differences in sister-chromatid alignment and compared the results with those obtained for nuclei of higher endopolyploidy levels. The number of FISH signals was taken as a measure for sister-chromatid alignment. An increase in the number of FISH signals with the ploidy level indicates the absence of cohesion at the loci under study. Furthermore, we tested by FISH sister-chromatid alignment at transgenic tandem repeat (lac operator) arrays, which have a tendency for increased somatic pairing in Arabidopsis. Additionally, we looked for possible correlations of positional sister-chromatid alignment with transcriptional activity and with the degree of overall DNA methylation.
MATERIALS AND METHODS
Plant material and preparation of nuclei:
Nuclei from young root tips or rosette leaves of A. thaliana accessions Columbia (Col) and Landsberg erecta (Ler) of the mutants ddm1 in the Col background (Vongs et al. 1993) and fwa-1 in the Ler background (Soppe et al. 2000) and of the transgenic line EL702C in the Col background (Kato and Lam 2001) were isolated after formaldehyde fixation and flow sorted on slides according to their ploidy level (2C–8C) as described (Pecinka et al. 2004). Nuclei of 16C and 32C were isolated from stems. Meristematic nuclei were prepared from roots of 2-day-old seedlings; ∼0.5-cm-long roots were incubated for 30 min in 100 μm bromodeoxyuridine (BrdU), 5 μm uridine, 0.1 μm fluorodeoxyuridine, fixed in 3:1 ethanol/acetic acid for at least 5 hr and washed three times for 20 min in 10 mm sodium citrate buffer (pH 4.8). After incubation for 35 min at 37° in 2% pectinase and 2% cellulase to soften the tissue, root tips were washed three times for 20 min in 10 mm sodium citrate buffer (pH 4.8), transferred to 45% acetic acid for 5–10 min, and squashed in a drop of 45% acetic acid. Coverslips were removed after freezing on dry ice. Then slides were immediately dehydrated in a series of 70, 90, and 96% ethanol, air dried, and stored at 4°.
Probe labeling and fluorescent in situ hybridization:
Bacterial artificial chromosomes (BACs) used for FISH were obtained from the Arabidopsis Biological Resource Center (Columbus, OH). The 178-bp A. thaliana centromere-specific sequence (pAL) was cloned by Martinez-Zapater et al. (1986). DNA of individual clones was isolated as described by Birnboim and Doly (1979).
DNA was labeled by nick translation with digoxigenin–dUTP, biotin–dUTP, or Cy3–dUTP according to Ward (2002). For painting of the chromosome 1 top arm, 15 pools of a total of 76 BACs (from T25K16 to T9G5) were labeled as described (Pecinka et al. 2004). Posthybridization washes and detection of FISH signals were performed according to Schubert et al. (2001). Biotin was detected by avidin conjugated with Texas Red (1:1000; Vector Laboratories, Burlingame, CA), goat-anti-avidin conjugated with biotin (1:200; Vector Laboratories), and again with avidin conjugated with Texas Red, digoxigenin by mouse-anti-digoxigenin (1:250; Roche), and goat-anti-mouse conjugated with Alexa-488 (1:200; Molecular Probes, Eugene, OR). Cy3 was observed directly. Nuclei and chromosomes were counterstained with DAPI (1 μg/ml) in Vectashield (Vector Laboratories). BrdU incorporation was detected by rat-anti-BrdU (1:100; Abcam) and rabbit-anti-rat conjugated with Cy3 (1:100; Jackson ImmunoResearch, West Grove, PA).
Microscopic evaluation, image processing, and statistics:
Analysis of fluorescence signals was performed with an epifluorescence microscope (Zeiss Axiophot) using a 100×/1.4 Zeiss plan apochromat objective and a Sony (DXC-950P) camera. Images were captured separately for each fluorochrome using the appropriate excitation and emission filters. The images were merged using Adobe Photoshop 6.0 (Adobe Systems) software. In 4C nuclei, the occurrence of three or four FISH signals was considered to represent sister-chromatid separation when their distance was larger than the signal diameter.
To distinguish 4C nuclei without BrdU incorporation from 2C nuclei in squashes from root-tip meristems, areas of BrdU-labeled S-phase nuclei were calculated on the basis of size measurements using a Digital Optical Microscope System (Schwertner GbR, Jena, Germany). Nuclei with an area larger than that of replicating, BrdU-labeled nuclei plus a 95% confidence interval were regarded as 4C. Fisher's exact test has been used to compare positional sister-chromatid separation frequencies.
RESULTS
In differentiated 4C nuclei, sister chromatids are separated with variably high frequencies at various positions but rarely along entire chromosome arm territories:
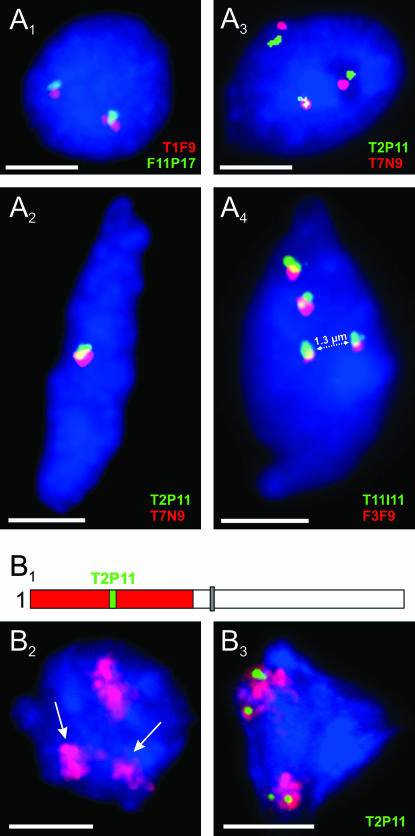
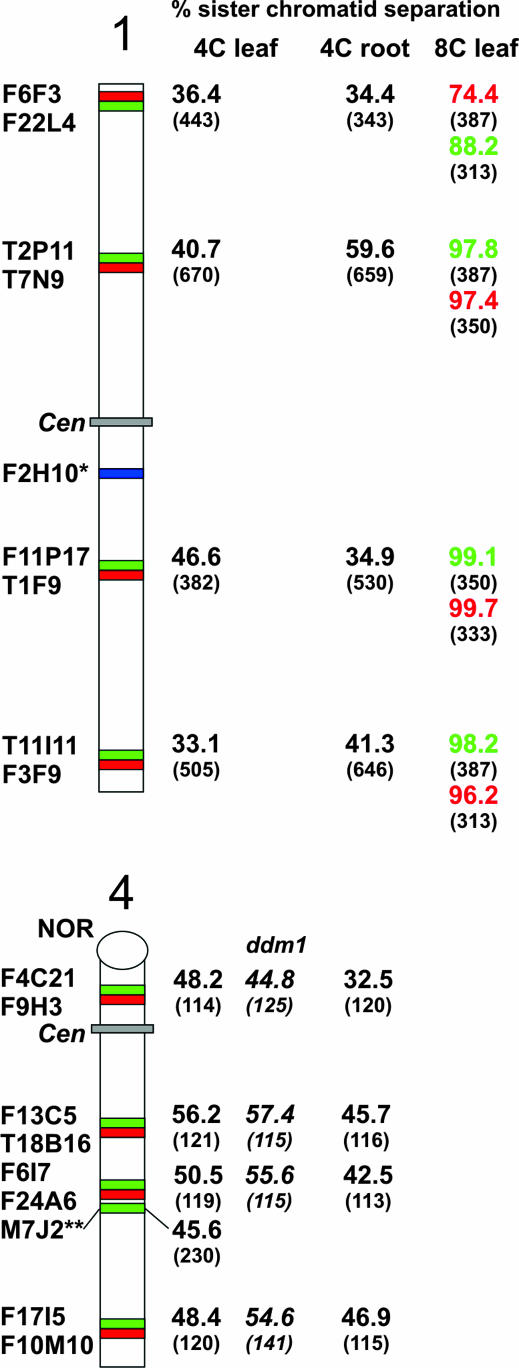
To analyze sister-chromatid alignment in A. thaliana interphase nuclei, individual BACs (or BAC pairs with inserts of adjacent genomic sequences) from different positions along chromosomes 1 and 4 were hybridized to flow-sorted 4C nuclei. One FISH signal (pairing of both homologs) or two FISH signals per BAC (or BAC pair) were regarded as positional alignment at the corresponding region, indicating that sister chromatids are aligned. Three or four signals were considered to indicate sister-chromatid separation (Figure 1). Positional sister-chromatid separation occurred in 32.5–59.6% of nuclei (Figure 2).
Figure 1.
Sister-chromatid alignment/separation of homologous ∼100-kb segments and of entire chromosome arms in 4C nuclei. (A1–A4) FISH with different BAC pairs. (A1) Sister-chromatid alignment at nonpaired homologous position. (A2) Sister-chromatid alignment at paired homologous positions. (A3) Sister-chromatid separation at one of the homologous positions. (A4) Positional sister-chromatid separation at both homologs. (B1) Scheme of chromosome 1 with the top arm labeled by a contig of BACs (red) and a 85.3-kbp sequence cloned in BAC T2P11 (green). (B2) Separation of the sister-chromatid arm territories of one homolog (arrows). (B3) Positional sister-chromatid separation (T2P11, green) within both homologous chromosome arm territories. Nuclei were counterstained with DAPI. Bars, 3 μm.
Figure 2.
Percentage of positional separation frequencies (number of investigated nuclei in parentheses) analyzed in accession Columbia and in the DNA hypomethylation mutant ddm1 in Col background after FISH with differentially labeled BACs from chromosomes 1 and 4. (*) F2H10 was tested only in 16C and 32C nuclei. (**) M7J2 was tested in 4C leaf nuclei of accession Ler (and of mutant fwa-1 in Ler background; for mutant data, see text). In 8C leaf nuclei, BACs were not hybridized as adjacent pairs but rather in various combinations. Only 4C leaf nuclei of the ddm1 mutant were studied.
At three positions of chromosome 1 (BAC pairs T2P11/T7N9, F11P17/T1F9, T11I11/F3F9) the separation frequencies differed significantly (P < 0.001) between leaf and root nuclei, suggesting a tissue-specific degree of sister-chromatid alignment. Even between individual midarm positions a significant (P < 0.001) variability of sister-chromatid alignment may occur in 4C root nuclei (e.g., between BAC pairs T2P11/T7N9 and F11P17/T1F9).
The ratio of 4C root nuclei with three or four signals varied for chromosome 1 positions between 1.1:1 (T2P11/T7N9) and 2.7:1 (F6F3-F22L4). The 1.1:1 ratio is significantly different (P < 0.001) from the random ratio of 2:1 (random separation involving one homolog should be twice as frequent as separation involving both homologs). The larger-than-expected proportion of nuclei with four signals might suggest a tendency for simultaneous separation of sister chromatids of both homologs at the respective homologous positions.
Using a FISH probe covering the top arm of chromosome 1, we analyzed the frequency of sister-chromatid alignment for the entire arm. While sister-chromatid separation at the segment corresponding to the BAC T2P11 within the top arm of chromosome 1 appeared in 38.1% of nuclei, completely separated sister-chromatid arm territories were found in only 6.1% of 359 4C leaf nuclei (Figure 1B). In 4.7% of nuclei one and in 1.4% both homologous arms were separated.
Neither DNA hypomethylation nor transcriptional activity impairs positional sister-chromatid alignment:
A comparison of sister-chromatid separation frequencies between 4C nuclei of wild type (accession Columbia) and of the hypomethylation mutant ddm1 (Vongs et al. 1993) in Col background displayed no significant differences at the same chromosomal positions of chromosome 4. Hence, sister-chromatid alignment is apparently not influenced by an overall DNA hypomethylation (Figure 2).
The flowering gene FWA residing in BAC M7J2 and mapped at the bottom arm of chromosome 4 is strongly methylated and not expressed in wild-type plants (accession Ler), but constitutively expressed and hypomethylated in leaf nuclei of the fwa-1 mutant in Ler background (Soppe et al. 2000). FISH signals of M7J2 revealed separation of sister chromatids in 45.6% of 4C leaf nuclei (n = 230) of wild-type Ler and in 33.9% of fwa-1 nuclei (n = 121) with a ratio of nuclei with three or four signals of 3.2:1 and 3.6:1, respectively. While the frequency of separation at the position of M7J2 in Ler nuclei is within the range observed for various positions in Col, it is even lower in fwa-1 mutant nuclei than in wild-type Ler nuclei where the FWA gene is silent. These results indicate the lack of an activation-mediated reduction of sister-chromatid alignment at the transcribed FWA locus.
Nuclei with an endopolyploidy level above 4C showed up to 100% sister-chromatid separation at the tested positions:
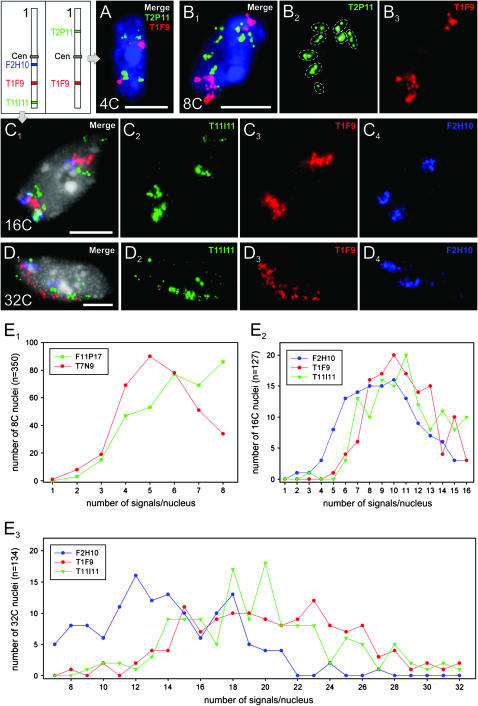
In 8C leaf nuclei, sister-chromatid separation varied between 74.4% and 99.7% along different positions of chromosome 1 (Figure 2). In 16C and 32C stem nuclei, BAC positions F2H10, T1F9, and T11I11 showed sister-chromatid separation in nearly all tested nuclei. From 8C nuclei it became obvious that alignment is retained more often at very terminal (see values for the BAC pair F6F3/F22L4 located 104 kbp upstream the telomeric repeats, Figure 2) than at interstitial arm positions.
In some 8C, 16C, and 32C nuclei, a number of separate FISH signals corresponding even to the maximum number of homologous chromatids was observed (Figure 3, B2 and E1, E2, and E3 ).
Figure 3.
Positional sister-chromatid alignment/separation as identified after FISH with BACs from different positions along chromosome 1. (A) Alignment vs. separation at BAC insert positions (T2P11 and T1F9) of both homologs in a 4C and (B1–B3) in an 8C leaf nucleus; note eight signal groups (circled) in B2. (C1–C4) Alignment/separation at a distal (T11I11), an intermediate (T1F9), and a proximal (F2H10) position of the chromosome 1 bottom arm in a 16C and (D1–D4) in a 32C nucleus. (E) Proportion of 8C, 16C, and 32C nuclei with varying numbers of FISH signals. (E1) Two BACs from the middle of the top and the bottom arm of chromosome 1 (Figure 2) showing a different degree of sister-chromatid alignment in 8C nuclei. (E2 and E3) The centromere-adjacent position F2H10 is more often aligned (i.e., showing fewer signals) than the other two positions in 16C (E2) and in 32C (E3) nuclei. Bars, 5 μm.
Sister-chromatid separation may vary along the same chromosome:
Simultaneous FISH with BACs from different positions along chromosome 1 has shown that sister chromatids in the same nucleus might be aligned at some and separated at other positions (Figure 3A).
In 8C nuclei, the degree of sister-chromatid alignment may vary also between midarm positions (Figure 3E1). In highly endopolyploid nuclei (32C), sister chromatids were on average more frequently separated in midarm and in distal than in pericentromeric positions (compare BACs F2H10, T1F9, and T11I11 in Figure 3, E2 and E3). Although it is difficult to count unambiguously individual signals in 16C and 32C nuclei, the high degree of separation becomes clearly evident from images such as shown in Figure 3D.
The majority of sister centromeres appear to be aligned up to a DNA content of 16C:
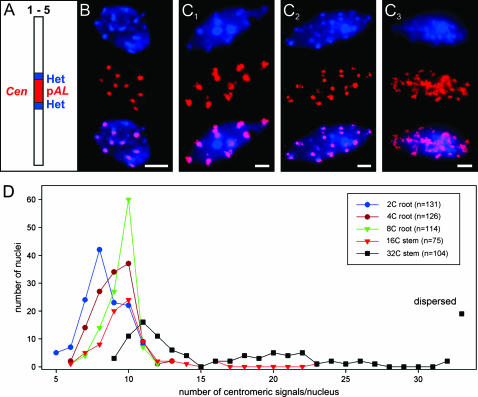
Most of the (tandem) repetitive sequences of A. thaliana occur around centromeres and at the NORs on the short arm termini of chromosomes 2 and 4. Therefore, up to 14 strongly DAPI-stained chromocenters per nucleus should appear if sister chromatids are aligned. However, because of frequent association of NORs with the corresponding pericentromeres (forming short-arm loops) and because of frequent association of homologous pericentromeric regions, nuclei from squashed organs revealed between 4 and 10 (most frequently 7 or 8) chromocenters (Fransz et al. 2002). To find out whether sister-chromatid alignment at centromeres is retained after endopolyploidization, we investigated flow-sorted nuclei of 4C, 8C (root), 16C, and 32C (stem) DNA content (Figure 4). After FISH with the tandem-repetitive centromere-specific 178-bp sequence (pAL) we found, compared with 2C nuclei, a shift toward more nuclei with up to 10 signals in 4C, 8C, and 16C nuclei. This shift indicates a lower degree of interchromosomal centromere associations, but not necessarily separation of sister chromatids. Of the 16C nuclei, 22.3% showed 11 or more centromeric signals; the same was true for 68.2% of 32C nuclei. Additionally, 18.3% of 32C nuclei yielded dispersed FISH signals with pAL (Figure 4C3). In 32C nuclei, these findings were paralleled by an increased number and/or diffuse appearance of DAPI-intense chromocenters. Thus, separation of sister centromeres is not evident up to an 8C DNA content, it appears to a low extent in 16C, and it increases strongly in 32C nuclei.
Figure 4.
Centromeric/pericentromeric sister-chromatid alignment. (A) Scheme of an A. thaliana chromosome with the 178-bp centromere-specific sequence (pAL) surrounded by pericentromeric heterochromatin that together form DAPI-intense chromocenters. (B) Alignment of centromeric regions in a 4C and (C1) in a 32C nucleus, (C2) partial separation/dispersion in a 32C nucleus, and (C3) extended centromere dispersion in a 32C nucleus as visualized by DAPI staining (top), FISH with pAL (middle), and after merging (bottom). Chromocenters not colocalizing with pAL signals represent NOR-specific heterochromatin of chromosomes 2 and 4. (D) Number of centromeric FISH signals in nuclei of different ploidy levels. From 4C to 8C, nuclei display mainly alignment of centromeres (≤10 pAL signals). In contrast, some 16C and the majority (86.5%) of 32C nuclei show clear separation, i.e., >10 centromeric signals, or signal dispersion. Bar, 5 μm.
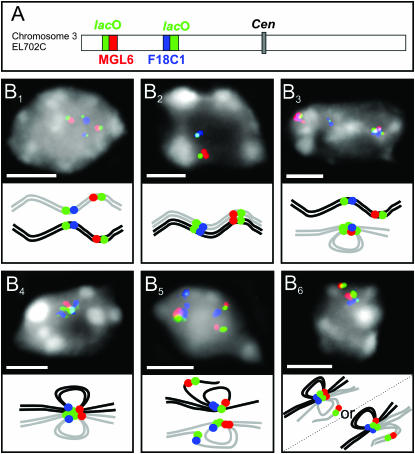
Sister-chromatid alignment is not increased at tandem repetitive lac operator array insertion sites:
In 2C leaf nuclei of homozygous transgenic plants with two insertion loci of lac operator (lac O) sequences on the top arm of chromosome 3 (line EL702C; Kato and Lam 2001), a significant increase of the somatic homologous pairing frequency of the transgene loci in comparison to that of average euchromatic regions was demonstrated (Pecinka et al. 2005). On the other hand, in Drosophila in which a regular development-specific somatic pairing occurs (Hiraoka et al. 1993), GFP-tagged lac O arrays allowed tracing of the separation of homologs and even of sister chromatids in male premeiotic mid-G2 nuclei (Vazquez et al. 2002). Therefore, we tested fixed 4C leaf nuclei of homozygous EL702C plants by FISH with lac O sequences and with BACs containing sequences that flank the lac O arrays as to the occurrence of sister-chromatid separation at or adjacent to the transgene loci (Figure 5). The two lac O loci at the chromosome 3 top arm may pair in an allelic as well as in an ectopic manner. Therefore, signal configurations, which cannot clearly be identified as alignment or separation, may appear (Figure 5B6). Configurations unambiguously indicating sister-chromatid separation (Figure 5B5) were identified at the lac O/MGL6 and at the lac O/F18C1 locus. Sister-chromatid separation was observed in 40.9% of each of the two loci in 44 nuclei. In addition, 20.5% and 18.2% equivocal configurations, respectively, were found (Figure 5B6). Thus, sister-chromatid separation at the lac O insertion sites occurred at about the same frequency as found for the different single-copy euchromatic positions in wild-type plants and is not counteracted by the tendency for homologous pairing at the tandem repeat loci. Life observation of GFP spots was not done because of uncertainties regarding the appearance of individual spots and their tendency to fuse with each other (Pecinka et al. 2005).
Figure 5.
Positional sister-chromatid alignment and/or separation at the lac O insertion sites in 4C leaf nuclei of A. thaliana line EL702C. (A) Scheme of chromosome 3 of line EL702C showing the lac O insertion sites (green) and the flanking regions MGL6 (red) and F18C1 (blue). (B) Selected images of nuclei after FISH with lac O, MGL6, and F18C1 with schematic interpretations below. (B1) Separation of homologous chromosomes but alignment of sister chromatids of both homologs. (B2) Allelic pairing and sister-chromatid alignment of both homologs. (B3) Ectopic pairing within one homolog and sister-chromatid alignment concerning both homologs. (B4) Allelic and ectopic pairing and sister-chromatid alignment of both homologs. (B5) Ectopic pairing and sister-chromatid separation at one insertion site on both homologs (unambiguous as to separation). (B6) Either allelic and ectopic pairing between both loci of both homologs and sister-chromatid separation at the distal locus of one homolog or allelic pairing between the proximal loci of both homologs, additional ectopic pairing involving one homolog, and sister-chromatid alignment of both homologs (ambiguous situation as to sister-chromatid separation; see text). Bars, 3 μm.
Positional sister-chromatid separation is also evident in meristematic cells:
Because leaf and root nuclei with a DNA content of 4C or higher are typical for differentiated cells with no need for further mitotic division, sister-chromatid cohesion might be dispensable in such nuclei. Therefore, we studied meristematic root-tip nuclei after incorporation of bromodeoxyuridine. Separation of sister chromatids at a distal position of the chromosome 1 top arm (F22L4) occurred in 30.9% of 55 replicating nuclei (Figure 6) and in 34.4% of 64 4C root-tip nuclei and hence was not significantly less frequent than in 4C nuclei of differentiated tissues.
Figure 6.
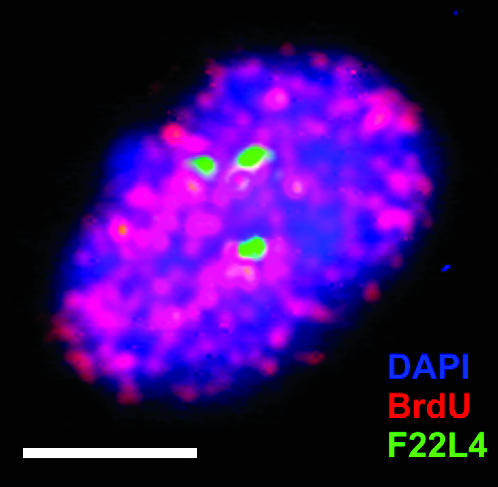
Positional separation of sister chromatids of one homolog at position F22L4 (green) of chromosome 1 in a replicating meristematic root nucleus after BrdU pulse labeling (red). Bar, 5 μm.
DISCUSSION
Our results show that FISH is effective to prove positional sister-chromatid separation as a common feature in S-phase, G2, and endopolyploid nuclei of A. thaliana.
Sister-chromatid separation is frequent, position-specific, and dynamic:
According to a model of Koshland and Guacci (2000), chromosome condensation toward nuclear division is mediated by “coalescence of cohesion sites” along paired sister chromatids. Between the coalescent sites symmetric loops are formed. The size of the presumed loops depends on the distance of cohesion sites and determines the degree of compaction. If the degree of compaction increases with genome size (Vinogradov 2005), larger genomes might have fewer cohesion sites and larger loops between them, possibly yielding positional separation along sister chromatids with a higher probability than, for instance, in budding yeast (Koshland and Guacci 2000). However, during prophase sister chromatids are continuously aligned and the distance between FISH signals on sister chromatids becomes very small or not resolvable by light microscopy. Assuming that the number and distance of cohesion sites along chromosome arms is not increased from replication up to mitosis, the distance of FISH signals for identical positions on sister chromatids in interphase nuclei should not exceed that observed during the highest compaction at metaphase if coalescence of cohesion sites were the main reason for mitotic chromosome condensation. However, frequently three or four FISH signals with distances of sometimes about half the nuclear diameter were observed in 4C nuclei. Recruitment of condensins toward mitosis might mediate an intense folding of sister-chromatid loops between cohesion sites, thus leading to the appearance of closely aligned sister chromatids of pro- and metaphase chromosomes (Haering and Nasmyth 2003; Hagstrom and Meyer 2003; Hirano 2005). Metaphase chromosomes with chromatid deletions mediated by erroneous repair can be observed after genotoxin exposure. Chromatin deleted from an interstitial position of one sister chromatid remains attached to the homologous region of its undamaged sister (see Figure 7 and Schubert et al. 1994). This observation confirms a closer proximity of identical sister chromatids during pro- and metaphase than during the preceding G2 when sister chromatids are frequently separated at various positions.
Figure 7.
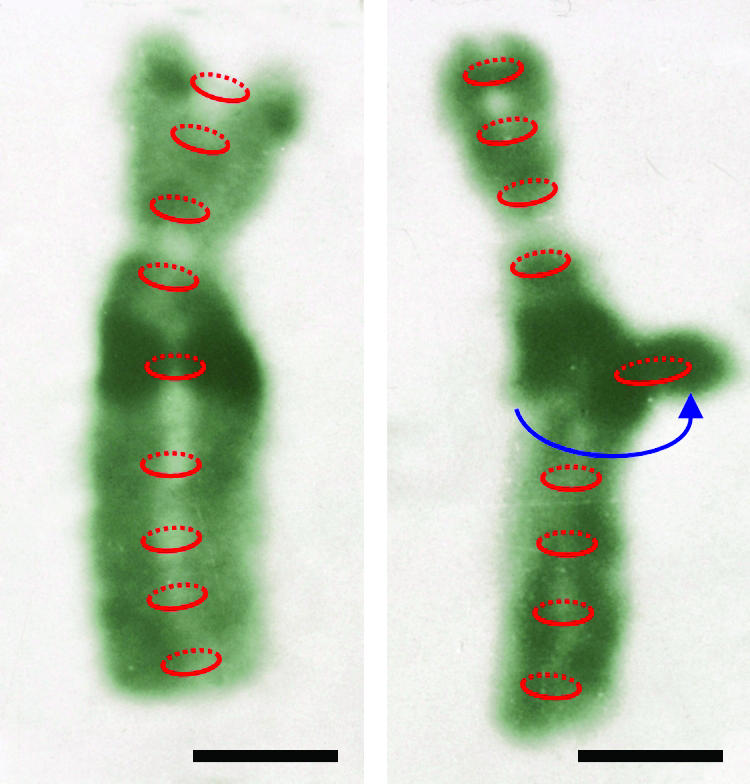
Presumed cohesion (red) along an undamaged metaphase chromosome of the field bean (left) and along a homolog with a circular interstitial chromatid deletion of (darkly stained) heterochromatic material from the left chromatid (right) imaged after Giemsa banding. The ring-shaped deleted chromatin remained attached to the homologous region of the undamaged sister chromatid (modified after Schubert et al. 1994). Bar, 1 μm.
The highest degree of alignment along interphase chromosome arms occurs around centromeres up to an endopolyploidy level of 16C. It remains an open question whether, for correct segregation during nuclear divisions, strict cohesion at centromeric regions is sufficient or whether a premitotic increase of homologous alignment along chromosome arms is required. Since even in late S-phase and in early G2 of meristematic cells positional sister-chromatid separation is evident, sparse cohesion along arms (and a somewhat more dense cohesion at telomeres) might be sufficient until the onset of prophase when condensins enforce sister-chromatid alignment. The observations from meristematic nuclei are in accordance with a tight connection of sister-chromatid exchange (requiring a close vicinity of homologous sister regions for recombination) with DNA replication when sister chromatids are just emerging.
In yeast, cohesion sites occupy the boundaries of transcriptionally silenced regions (Laloraya et al. 2000), are usually not transcribed (Tanaka et al. 1999), and shift away from transcribing regions (Glynn et al. 2004; Lengronne et al. 2004). In comparison to Arabidopsis wild-type nuclei, constitutive expression of the FWA gene in fwa-1 mutant nuclei did not cause increased sister-chromatid separation at this locus, indicating that there is no cohesion site in the vicinity to interfere with transcription at this locus. Further comparative studies with other gene regions in silent vs. active state are needed for conclusions as to whether or not transcriptional activity has an impact on cohesion in Arabidopsis.
In living G2 spermatocytes of Drosophila, GFP tagging revealed positional sister-chromatid separation at lac O loci (Vazquez et al. 2002). Similarly, sister-chromatid separation at transgenic tandem repeat loci of 4C nuclei of Arabidopsis appears with a “normal” frequency, albeit these loci are more often homologously paired than average euchromatic regions are (Pecinka et al. 2005). While somatic pairing frequency of lac O repeat arrays decreases with decreasing methylation at CpG sites (Watanabe et al. 2005), overall sister-chromatid alignment is not significantly less frequent in the background of the DNA hypomethylation mutant ddm1 than in 4C wild-type nuclei.
Reproducibly different sister-chromatid alignment frequencies along chromosome arms suggest a nonuniform distribution of cohesion sites that, at least in part, may differ between tissues, indicating a developmentally regulated dynamics of sister-chromatid cohesion (Figures 2 and 8).
Figure 8.
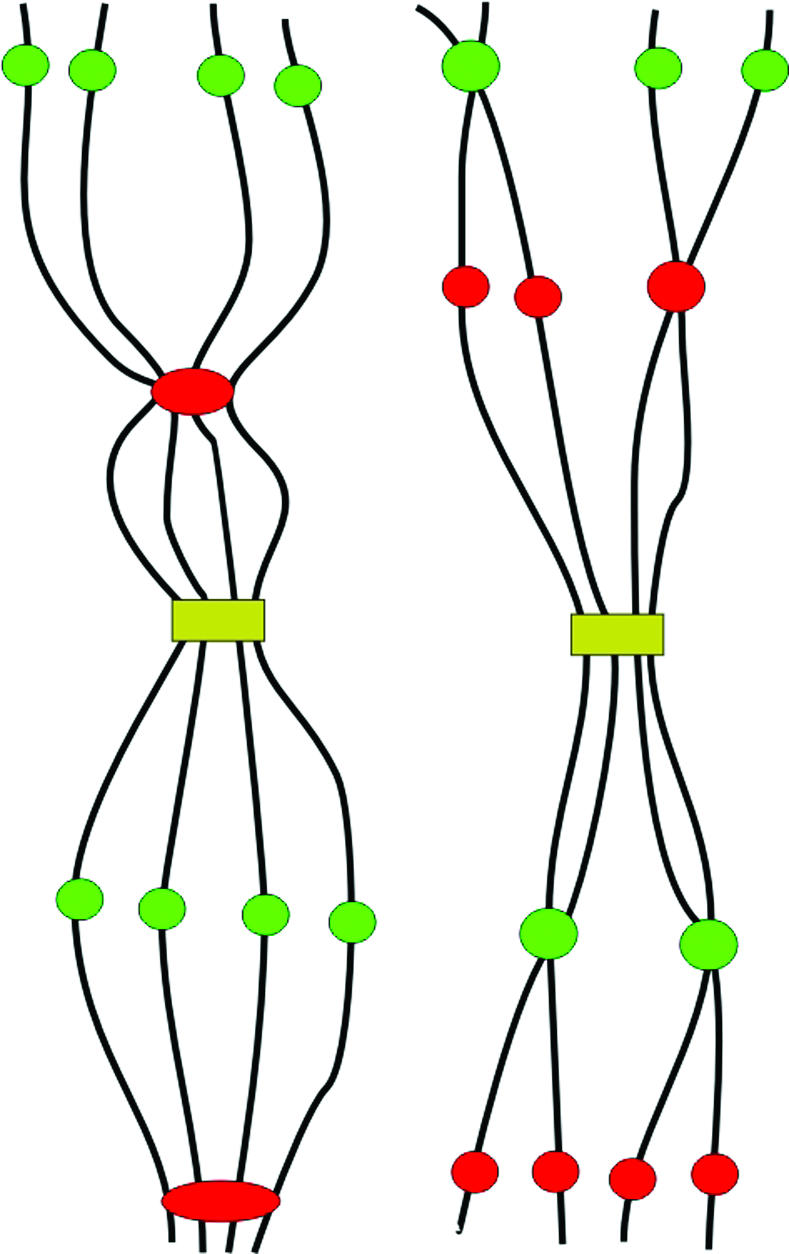
Various possibilities of sister-chromatid alignment/separation at different positions along chromosomes of an 8C nucleus.
Separation of identical chromatids increases with the endopolyploidy level:
Contrary to the situation observed in 4C nuclei, positional sister-chromatid separation outside the pericentromeres may involve up to 100% of >4C nuclei. This observation contradicts the results of Esch et al. (2003). These authors reported a very high association frequency of homologous positions in endopolyploid A. thaliana nuclei, resulting in only one GFP spot per nucleus after lac operator/GFP-lac repressor chromatin tagging. However, transgenic repeat loci have a strong tendency for homologous pairing, which is not typical for average euchromatic regions (Pecinka et al. 2004, 2005). Sister centromeres tend to separate from the 16C level on, in stem nuclei. Individual ∼100-kb regions are of a spread appearance within highly endopolyploid nuclei (Figure 3, B2, D2, and D3). In general, the degree of positional alignment decreases with increasing ploidy level (as to be expected when chromatids do not form polytene chromosomes) and is lower at interstitial than at centromeric or terminal positions. Nondividing endopolyploid nuclei are less endangered by unrepaired double-strand breaks than are dividing cells; therefore, nuclei may possibly possess or acquire fewer cohesion sites with increasing endopolyploidy level. Whether the lower degree of sister-chromatid alignment correlates with a higher level of transcription in endopolyploid nuclei requires further investigation. However, at least our data obtained for the FWA gene are not in favor of a transcription-mediated reduction of sister-chromatid alignment.
Acknowledgments
We thank Eric Lam for allowing us to use the Arabidopsis line EL702C, Rigomar Rieger and Frank Uhlmann for stimulating discussions, and Joachim Bruder, Martina Kühne, and Rita Schubert for excellent technical assistance.
References
- Biggins, S., and A. W. Murray, 1999. Sister chromatid cohesion in mitosis. Curr. Opin. Genet. Dev. 9: 230–236. [DOI] [PubMed] [Google Scholar]
- Birnboim, H. C., and J. Doly, 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7: 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blat, Y., and N. Kleckner, 1999. Cohesion bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell 98: 249–259. [DOI] [PubMed] [Google Scholar]
- Cai, X., F. G. Dong, R. E. Edelmann and C. A. Makaroff, 2003. The Arabidopsis SYN1 cohesin protein is required for sister chromatid arm cohesion and homologous chromosome pairing. J. Cell Sci. 116: 2999–3007. [DOI] [PubMed] [Google Scholar]
- Campbell, J. L., and O. Cohen-Fix, 2002. Chromosome cohesion: Ring around the sisters? Trends Biochem. Sci. 27: 492–495. [DOI] [PubMed] [Google Scholar]
- D'Amours, D., F. Stegmeier and A. Amon, 2004. Cdc14 and condensin control the dissolution of cohesin-independent chromosome linkages at repeated DNA. Cell 117: 455–469. [DOI] [PubMed] [Google Scholar]
- Dong, F., X. Cai and C. A. Makaroff, 2001. Cloning and characterization of two Arabidopsis genes that belong to the RAD21/REC8 family of chromosome cohesin proteins. Gene 271: 99–108. [DOI] [PubMed] [Google Scholar]
- Dynek, J. N., and S. Smith, 2004. Resolution of sister telomere association is required for progression through mitosis. Science 304: 97–100. [DOI] [PubMed] [Google Scholar]
- Esch, J. J., M. Chen, M. Sanders, M. Hillestad, S. Ndkium et al., 2003. A contradictory GLABRA3 allele helps define gene interactions controlling trichome development in Arabidopsis. Development 130: 5885–5894. [DOI] [PubMed] [Google Scholar]
- Fransz, P., J. H. de Jong, M. Lysak, M. Ruffini Castiglione and I. Schubert, 2002. Interphase chromosomes in Arabidopsis are organized as well defined chromocenters from which euchromatin loops emanate. Proc. Natl. Acad. Sci. USA 99: 14584–14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn, E. F., P. C. Megee, H. G. Yu, C. Mistrot, E. Unal et al., 2004. Genome-wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae. PLoS Biol. 2: 1325–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guacci, V., E. Hogan and D. Koshland, 1994. Chromosome condensation and sister chromatid pairing in budding yeast. J. Cell Biol. 125: 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haering, C. H., and K. Nasmyth, 2003. Building and breaking bridges between sister chromatids. BioEssays 25: 1178–1191. [DOI] [PubMed] [Google Scholar]
- Hagstrom, K. A., and B. J. Meyer, 2003. Condensin and cohesin: more than chromosome compactor and glue. Nat. Rev. Genet. 4: 520–534. [DOI] [PubMed] [Google Scholar]
- Hirano, T., 2000. Chromosome cohesion, condensation, and separation. Annu. Rev. Biochem. 69: 115–144. [DOI] [PubMed] [Google Scholar]
- Hirano, T., 2005. Condensins: organizing and segregating the genome. Curr. Biol. 15: R265–R275. [DOI] [PubMed] [Google Scholar]
- Hiraoka, Y., A. F. Dernburg, S. J. Parmelee, M. C. Rykowski, D. A. Agard et al., 1993. The onset of homologous chromosome pairing during Drosophila melanogaster embryogenesis. J. Cell Biol. 120: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger, R., 2003. SMC proteins at the crossroads of diverse chromosomal processes. IUBMB Life 55: 643–652. [DOI] [PubMed] [Google Scholar]
- Kato, N., and E. Lam, 2001. Detection of chromosome tagged with green fluorescent protein in live Arabidopsis thaliana plants. Genome Biol. 2: research0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, N., and E. Lam, 2003. Chromatin of endoreduplicated pavement cells has greater range of movement than that of diploid guard cells in Arabidopsis thaliana. J. Cell Sci. 116: 2195–2201. [DOI] [PubMed] [Google Scholar]
- Kim, J.-S., T. B. Krasieva, V. LaMorte, A. M. R. Taylor and K. Yokomori, 2002. Specific recruitment of human cohesion to laser-induced DNA damage. J. Biol. Chem. 277: 45149–45153. [DOI] [PubMed] [Google Scholar]
- Koshland, D. E., and V. Guacci, 2000. Sister chromatid cohesion: the beginning of a long and beautiful relationship. Curr. Opin. Cell Biol. 12: 297–301. [DOI] [PubMed] [Google Scholar]
- Laloraya, S., V. Guacci and D. Koshland, 2000. Chromosomal addresses of the cohesin component Mcd1p. J. Cell Biol. 151: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, W. S., X. Yang and C. A. Makaroff, 2005. Characterization of Arabidopsis thaliana SMC1 and SMC3: evidence that AtSMC3 may function beyond chromosome cohesion. J. Cell Sci. 118: 3037–3048. [DOI] [PubMed] [Google Scholar]
- Lee, J. Y., and T. L. Orr-Weaver, 2001. The molecular basis of sister-chromatid cohesion. Annu. Rev. Cell Dev. Biol. 17: 753–777. [DOI] [PubMed] [Google Scholar]
- Lengronne, A., Y. Katou, S. Mori, S. Yokobayashi, G. P. Kelly et al., 2004. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature 430: 573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. M., J. McElver, I. Tzafrir, R. Joosen, P. Wittich et al., 2002. Condensin and cohesin knockouts in Arabidopsis exhibit a titan seed phenotype. Plant J. 29: 405–415. [DOI] [PubMed] [Google Scholar]
- Maguire, M. P., 1990. Sister chromatid cohesiveness: vital function, obscure mechanism. Biochem. Cell Biol. 68: 1231–1242. [DOI] [PubMed] [Google Scholar]
- Martinez-Zapater, J. M., M. A. Estelle and C. R. Sommerville, 1986. A highly repeated DNA sequence in Arabidopsis thaliana. Mol. Gen. Genet. 204: 4417–4423. [Google Scholar]
- Megee, P. C., C. Mistrot, V. Guacci and D. Koshland, 1999. The centromeric sister chromatid cohesion site directs Mcd1p binding to adjancent sequences. Mol. Cell 4: 445–450. [DOI] [PubMed] [Google Scholar]
- Mercier, R., D. Vezon, E. Bullier, J. C. Motamayor, A. Sellier et al., 2001. SWITCH1 (SWI1): a novel protein required for the establishment of sister chromatid cohesion and for bivalent formation at meiosis. Genes Dev. 15: 1859–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier, R., S. J. Armstrong, C. Horlow, N. P. Jackson, C. A. Makaroff et al., 2003. The meiotic protein SWI1 is required for axial element formation and recombination initiation in Arabidopsis. Development 130: 3309–3318. [DOI] [PubMed] [Google Scholar]
- Miyazaki, W. Y., and T. L. Orr-Weaver, 1994. Sister-chromatid cohesion in mitosis and meiosis. Annu. Rev. Genet. 28: 167–187. [DOI] [PubMed] [Google Scholar]
- Morrison, C., P. Vagnarelli, E. Sonoda, S. Takeda and W. C. Earnshaw, 2003. Sister chromatid cohesion and genome stability in vertebrate cells. Biochem. Soc. Trans. 31: 263–265. [DOI] [PubMed] [Google Scholar]
- Nasmyth, K., 2005. How might cohesin hold sister chromatids together? Philos. Trans. R. Soc. Lond. B Biol. Sci. 360: 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth, K., and A. Schleiffer, 2004. From a single double helix to paired double helices and back. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecinka, A., V. Schubert, A. Meister, G. Kreth, M. Klatte et al., 2004. Chromosome territory arrangement and homologous pairing in nuclei of Arabidopsis thaliana are predominantly random except for NOR-bearing chromosomes. Chromosoma 113: 258–269. [DOI] [PubMed] [Google Scholar]
- Pecinka, A., N. Kato, A. Meister, A. V. Probst, I. Schubert et al., 2005. Tandem repetitive transgenes and fluorescent chromatin tags alter the local interphase chromosome arrangement in Arabidopsis thaliana. J. Cell Sci. 118: 3751–3758. [DOI] [PubMed] [Google Scholar]
- Pereira, G., and E. Schiebel, 2004. Cdc14 phosphatase resolves the rDNA segregation delay. Nat. Cell Biol. 6: 473–475. [DOI] [PubMed] [Google Scholar]
- Revenkova, E., M. Eijpe, C. Heyting, C. A. Hodges, P. A. Hunt et al., 2004. Cohesion SMC1β is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat. Cell Biol. 6: 555–562. [DOI] [PubMed] [Google Scholar]
- Riedel, C. G., J. Gregan, S. Gruber and K. Nasmyth, 2004. Is chromatin remodeling required to build sister-chromatid cohesion? Trends Biochem. Sci. 29: 389–392. [DOI] [PubMed] [Google Scholar]
- Rieder, C. L., and R. Cole, 1999. Chromatid cohesion during mitosis: lessons from meiosis. J. Cell Sci. 112: 2607–2613. [DOI] [PubMed] [Google Scholar]
- Schubert, I., R. Rieger, J. Fuchs and U. Pich, 1994. Sequence organization and the mechanism of interstitial deletion clustering in a plant genome (Vicia faba). Mutat. Res. 325: 1–5. [DOI] [PubMed] [Google Scholar]
- Schubert, I., P. F. Fransz, J. Fuchs and J. H. de Jong, 2001. Chromosome painting in plants. Methods Cell Sci. 23: 57–69. [PubMed] [Google Scholar]
- Soppe, W. J. J., S. E. Jacobsen, C. Alonso-Blanco, J. P. Jackson, T. Kakutani et al., 2000. The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol. Cell 6: 791–802. [DOI] [PubMed] [Google Scholar]
- Ström, L., H. B. Lindroos, K. Shirahige and C. Sjögren, 2004. Postreplicative recruitment of cohesin to double-strand breaks is required for DNA repair. Mol. Cell 16: 1003–1015. [DOI] [PubMed] [Google Scholar]
- Sullivan, M., T. Higuchi, V. L. Katis and F. Uhlmann, 2004. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell 117: 471–482. [DOI] [PubMed] [Google Scholar]
- Tanaka, T., M. P. Cosma, K. Wirth and K. Nasmyth, 1999. Identification of cohesion association sites at centromeres and along chromosome arms. Cell 98: 847–858. [DOI] [PubMed] [Google Scholar]
- Tang, Z. Y., Y. X. Sun, S. E. Harley, H. Zou and H. T. Yu, 2004. Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc. Natl. Acad. Sci. USA 101: 18012–18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann, F., 2003. Chromosome cohesion and separation: from men and molecules. Curr. Biol. 13: R104–R114. [DOI] [PubMed] [Google Scholar]
- Uhlmann, F., 2004. The mechanism of sister chromatid cohesion. Exp. Cell Res. 296: 80–85. [DOI] [PubMed] [Google Scholar]
- Ünal, E., A. Arbel-Eden, U. Sattler, R. Shroff, M. Lichten et al., 2004. DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesin domain. Mol. Cell 16: 991–1002. [DOI] [PubMed] [Google Scholar]
- van Heemst, D., and C. Heyting, 2000. Sister chromatid cohesion and recombination in meiosis. Chromosoma 109: 10–26. [DOI] [PubMed] [Google Scholar]
- Vazquez, J., A. S. Belmont and J. W. Sedat, 2002. The dynamics of homologous chromosome pairing during male Drosophila meiosis. Curr. Biol. 12: 1473–1483. [DOI] [PubMed] [Google Scholar]
- Vinogradov, A. E., 2005. Genome size and chromatin condensation in vertebrates. Chromosoma 113: 362–369. [DOI] [PubMed] [Google Scholar]
- Volpi, E. V., D. Sheer and F. Uhlmann, 2001. Cohesion, but not too close. Curr. Biol. 11: R378. [DOI] [PubMed] [Google Scholar]
- Vongs, A., T. Kakutani, R. A. Martienssen and E. J. Richards, 1993. Arabidopsis thaliana DNA methylation mutants. Science 260: 1926–1928. [DOI] [PubMed] [Google Scholar]
- Ward, P. B., 2002. FISH probes and labelling techniques, pp. 5–28 in FISH: A Practical Approach (Practical Approach Series: 260), edited by B. Beatty, S. Mai and J. Squire. Oxford University Press, Oxford.
- Warren, W. D., S. Steffensen, E. Lin, P. Coelho, M.-L. Loupart et al., 2000. The Drosophila RAD21 cohesin persists at the centromere region in mitosis. Curr. Biol. 10: 1463–1466. [DOI] [PubMed] [Google Scholar]
- Watanabe, Y., 2005. Sister chromatid cohesion along arms and at centromeres. Trends Genet. 21: 405–412. [DOI] [PubMed] [Google Scholar]
- Watanabe, K., A. Pecinka, A. Meister, I. Schubert and E. Lam, 2005. DNA hypomethylation reduces homologous pairing of inserted tandem repeat arrays in somatic nuclei of Arabidopsis thaliana. Plant J. 44: 531–540. [DOI] [PubMed] [Google Scholar]
- Webber, H. A., L. Howard and S. E. Bickel, 2004. The cohesion protein ORD is required for homologue bias during meiotic recombination. J. Cell Biol. 164: 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalon, M., S. Gal, Y. Segev, S. Selig and K. L. Skorecki, 2004. Sister chromatid separation at human telomeric regions. J. Cell Sci. 117: 1961–1970. [DOI] [PubMed] [Google Scholar]