Abstract
Our previous work suggests that the Nhp6 HMGB protein stimulates RNA polymerase II transcription via the TATA-binding protein TBP and that Nhp6 functions in the same functional pathway as the Gcn5 histone acetyltransferase. In this report we examine the genetic relationship between Nhp6 and Gcn5 with the Mot1 and Ccr4–Not complexes, both of which have been implicated in regulating DNA binding by TBP. We find that combining either a nhp6ab or a gcn5 mutation with mot1, ccr4, not4, or not5 mutations results in lethality. Combining spt15 point mutations (in TBP) with either mot1 or ccr4 also results in either a growth defect or lethality. Several of these synthetic lethalities can be suppressed by overexpression of TFIIA, TBP, or Nhp6, suggesting that these genes facilitate formation of the TBP–TFIIA–DNA complex. The growth defect of a not5 mutant can be suppressed by a mot1 mutant. HO gene expression is reduced by nhp6ab, gcn5, or mot1 mutations, and the additive decreases in HO mRNA levels in nhp6ab mot1 and gcn5 mot1 strains suggest different modes of action. Chromatin immunoprecipitation experiments show decreased binding of TBP to promoters in mot1 mutants and a further decrease when combined with either nhp6ab or gcn5 mutations.
TRANSCRIPTIONAL activation by RNA polymerase II (pol II) requires the assembly of a complex of general transcription factors at a promoter (Hampsey 1998; Dvir et al. 2001). It is believed that transcriptional coactivators function by stimulating DNA binding by the general transcription factors TBP (TATA-binding protein), TFIIA, and TFIIB. Additionally, there are transcription factors that have been shown to negatively regulate binding of TBP to promoter DNA (reviewed by Lee and Young 1998; reviewed by Pugh 2000). Factors such as TAF1 and NC2 interact with TBP and inhibit its activity (Goppelt et al. 1996; Mermelstein et al. 1996; Bai et al. 1997; Kokubo et al. 1998). In contrast, Mot1 can disassociate TBP from DNA (Auble et al. 1994), and the Ccr4–Not complex may inhibit the recruitment of other general factors by TBP (Collart 1996; Badarinarayana et al. 2000). Additionally, two TBP molecules can dimerize to create a form that does not bind DNA (Coleman and Pugh 1997).
Mot1 is thought to inhibit transcription of certain genes by inhibiting TBP binding (for review see Pereira et al. 2003). In vitro, the Mot1 protein binds to TBP–DNA complexes and uses the energy of ATP to dissociate TBP from the DNA (Auble et al. 1994; Darst et al. 2003). Mot1 is an essential gene, and the fact that mot1 mutations cause derepression of specific genes is consistent with a proposed role as a negative regulator (Auble et al. 1994). However, Mot1 also functions as a positive regulator of transcription, as mot1 mutations reduce expression of certain genes (Andrau et al. 2002; Dasgupta et al. 2002). There are strong genetic interactions between MOT1 and SPT15 (encoding TBP) and with other basal factors, including TOA1 and TOA2 (encoding TFIIA), SPT3, and the Ccr4–Not complex (Collart 1996; Madison and Winston 1997). It has been suggested that Mot1 can stimulate transcription by inhibiting the association of NC2, a TBP inhibitor, with promoters (Geisberg et al. 2002) and that the Mot1–TBP complex delivers TBP to TAF-independent genes (Gumbs et al. 2003). Additionally, Mot1 is required for nucleosome remodeling at the GAL1 promoter (Topalidou et al. 2004). In normally growing cells, Mot1 co-occupies promoters with TBP, but not with TFIIB, TFIIA, or TAFs (Geisberg and Struhl 2004).
The Ccr4–Not complexes have multiple roles in gene regulation, including regulation of transcriptional initiation, elongation, and mRNA degradation (for reviews see Collart 2003; for reviews see Denis and Chen 2003). Ccr4–Not has been implicated as both a positive and a negative regulator of transcription (Liu et al. 1998), and the Gcn4 DNA-binding activator can recruit Ccr4–Not to promoters (Swanson et al. 2003). Some of the genes encoding subunits of this protein complex have been found to interact both physically and genetically with TBP, TAFs, and regulators of TBP binding, and it has been suggested that Ccr4–Not represses transcription by inhibiting DNA binding by TBP (Collart 1996; Badarinarayana et al. 2000; Lemaire and Collart 2000; Deluen et al. 2002).
We have studied the regulation of the yeast HO gene, and our studies suggest that Gcn5 and Nhp6 function in parallel to activate expression of this gene (Yu et al. 2000, 2003). Gcn5 is the histone acetyltransferase present in the yeast SAGA complex, and histone acetylation by Gcn5 is required for expression of many yeast genes (Sterner and Berger 2000). Nhp6 is related to the HMGB family of small, abundant chromatin proteins that bend DNA sharply and modulate gene expression (Travers 2003). Nhp6 is encoded by two genes, NHP6A and NHP6B. No phenotype is seen in nhp6a and nhp6b single mutants, while the nhp6a nhp6b double mutant (which we will describe as nhp6ab) is temperature sensitive for growth (Costigan et al. 1994) and shows transcriptional defects (Paull et al. 1996; Yu et al. 2000; Fragiadakis et al. 2004). Nhp6 also functions with Spt16 and Pob3, as part of the yeast FACT complex, to promote transcriptional elongation (Formosa et al. 2001), and Nhp6 is important for expression of the SNR6 gene, transcribed by RNA polymerase III (Kruppa et al. 2001; Lopez et al. 2001; Martin et al. 2001).
Our data suggest that Gcn5 and Nhp6 function to promote assembly of the TBP–TFIIA–DNA complex (Yu et al. 2003; Biswas et al. 2004; Eriksson et al. 2004a). Viable mutations affecting TBP or TFIIA (spt15 or toa2, respectively) are lethal in gcn5 or nhp6ab mutant strains. TBP overexpression suppresses the temperature-sensitive growth defect of nhp6ab strains and certain transcriptional defects of either nhp6ab or gcn5 mutants. Additionally, the gcn5 nhp6ab triple mutant displays a strong synthetic growth defect, but this phenotype can be suppressed by mutations in the SPT3 gene. Spt3, which is part of the SAGA complex with Gcn5 (Sterner et al. 1999), interacts with TBP both physically and genetically (Eisenmann et al. 1992). We find that an spt3 mutation can suppress a number of gcn5 and nhp6ab defects, including reduced HO expression, temperature-sensitive growth, and synthetic lethality with TBP mutants. Chromatin immunoprecipitation experiments show that Spt3 regulates TBP binding in vivo, inhibiting TBP binding to the HO promoter while stimulating TBP binding to GAL1 (Dudley et al. 1999; Yu et al. 2003).
In this study we use genetic tools to examine the relationship of Mot1 and Ccr4–Not to Gcn5 and Nhp6. Spt3, Mot1, and Ccr4–Not all regulate binding of TBP to DNA, and spt3 mutations suppress many gcn5 and nhp6ab defects. However, instead of suppression, we find synthetic lethal interactions between Mot1 and Ccr4–Not with Gcn5 and Nhp6. Multicopy suppression experiments support a critical role of these factors in facilitating formation of the TBP–TFIIA complex on DNA. Additive effects on HO gene transcription suggest that Mot1 functions differently from either Nhp6 or Gcn5. Chromatin immunoprecipitation (ChIP) experiments show that TBP binding to promoters is reduced in mot1 mutants, with an additive decrease when combined with nhp6ab or gcn5.
MATERIALS AND METHODS
Strains and media:
All yeast strains used are listed in Table 1 and are isogenic in the W303 background (Thomas and Rothstein 1989). Standard genetic methods were used for strain construction (Rothstein 1991; Sherman 1991). W303 strains with disruptions in gcn5, nhp6a, and nhp6b have been described (Yu et al. 2000, 2003) and the mot1(R1243I) allele was identified in a screen for Spt− mutations (Jiang and Stillman 1996). The ccr4 disrupted strain was provided by Clyde Denis, and the not4 and not5 disrupted strains by Martine Collart. These strains were then crossed to generate the strains used here. Strain DY9384 was constructed by disrupting the LYS2 gene in strain DY9348 with the D588 lys2∷HIS3 marker swap plasmid (Voth et al. 2003). Cells were grown at the indicated temperature in YEPD medium (Sherman 1991), except where synthetic complete medium with 2% glucose supplemented with adenine, uracil, and amino acids, as appropriate, but lacking essential components, was used to select for plasmids. 5-FOA medium was prepared as described (Boeke et al. 1984).
TABLE 1.
Strain list
| DY 150 | MATa | ade2 can1 his3 leu2 trp1 ura3 |
| DY5265 | MATa | gcn5∷TRP1 ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY7139 | MATa | nhp6a∷KanMX nhp6b∷ADE2 ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY7176 | MATa | ccr4∷LEU2 ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY7441 | MATa | ccr4∷LEU2 nhp6a∷KanMX nhp6b∷ADE2 NHP6B(YCp-URA3) ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY7462 | MATa | mot1(R1243I) ade2 can1 his3 leu2 trp1 ura3 |
| DY7463 | MATα | mot1(R1243I) ade2 can1 his3 leu2 trp1 ura3 |
| DY7841 | MATa | gcn5∷TRP1 mot1(R1243I) ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY7847 | MATa | mot1(R1243I) nhp6a∷KanMX nhp6b∷ADE2 ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY8237 | MATa | mot1(R1243I) spt15∷LEU2 SPT15(YCp-URA3) ade2 can1 his3 leu2 trp1 ura3 |
| DY8563 | MATa | ccr4∷LEU2 gcn5∷TRP1 GCN5(YCp-URA3) ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY8617 | MATα | not4∷LEU2 ade2 can1 his3 leu2 trp1 ura3 |
| DY8618 | MATa | gcn5∷HIS3 not4∷LEU2 GCN5(YCp-URA3) ade2 can1 his3 leu2 trp1 ura3 |
| DY8625 | MATa | nhp6a∷KanMX nhp6b∷ADE2 not5∷LEU2 NHP6A(YCp-URA3) ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY8626 | MATa | not5∷LEU2 ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY8627 | MATα | not5∷LEU2 ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY8628 | MATa | gcn5∷HIS3 not5∷LEU2 GCN5(YCp-URA3) ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY9348 | MATa | ccr4∷LEU2 spt15∷ADE2 SPT15(YCp-URA3) ade2 can1 his3 leu2 trp1 ura3 |
| DY9383 | MATa | mot1(R1243I) spt15∷LEU2 SPT15(YCp-URA3) ade2 can1 leu2 lys2 trp1 ura3 |
| DY9384 | MATa | ccr4∷LEU2 spt15∷ADE2 SPT15(YCp-URA3) ade2 can1 leu2 lys2 trp1 ura3 |
| DY9470 | MATa | ccr4∷LEU2 mot1(R1243I) ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY9545 | MATa | mot1(R1243I) not4∷LEU2 ade2 can1 his3 leu2 lys2 trp1 ura3 |
| DY9582 | MATa | mot1(R1243I) not5∷LEU2 ade2 can1 his3 leu2 lys2 trp1 ura3 |
Plasmids:
The multicopy plasmids used are listed in Table 2. Plasmid M4252 was constructed by moving a 1.8-kb SacI–XhoI fragment with GCN5 from plasmid pRS315–GCN5 (Marcus et al. 1994) into pRS425 (Christianson et al. 1992). Plasmid M4462 was constructed by moving a 0.95-kb HindIII–SacI fragment with NHP6A from plasmid M4221 (Biswas et al. 2004) into pRS325 (Sikorski and Hieter 1989). A 2.25-kb BamHI–PstI fragment with SPT15 from pSH2223 (Yu et al. 2003) was cloned into YEplac195 (Gietz and Sugino 1988) and pRS425 (Christianson et al. 1992), constructing M4403 and M4480, respectively. Plasmid M2661 was isolated from a YCp50 genomic library as complementing the temperature-sensitive phenotype of the mot1 mutant (Jiang and Stillman 1996), and M2719 was constructed from M2661 by deleting a 3.5-kb BamHI fragment within the MOT1 gene. The mot1(R1243I) allele was cloned by transforming DY7463 [mot1(R1243I)] with plasmid M2719, which had been cleaved with SacI, yielding plasmid M5099, which was then sequenced.
TABLE 2.
Plasmids
| Plasmid | Description | Source |
|---|---|---|
| pRS425 | YEp–LEU2 vector | Christianson et al. (1992) |
| pRS327 | YEp–LYS2 vector | Eriksson et al. (2004b) |
| YEplac195 | YEp–URA3 vector | Gietz and Sugino (1988) |
| pRS314 | YCp–TRP1 vector | Sikorski and Hieter (1989) |
| M2661 | 26-kb genomic fragment with MOT1 in YCp50 | Jiang and Stillman (1996) |
| M2719 | M2661 with 3.5-kb BamHI fragment deleted | This work |
| M5099 | mot1(R1243I) in YCp50 | This work |
| M4252 | GCN5 in YEp–LEU2 plasmid | This work |
| M3000 | MOT1 in YCp–URA3 plasmid | Jiang and Stillman (1996) |
| M4462 | NHP6A in YEp–LEU2 plasmid | This work |
| M4797 | NHP6A in YEp–LYS2 plasmid | Biswas et al. (2004) |
| M4221 | NHP6A in YEp–URA3 plasmid | Biswas et al. (2004) |
| pRS426–SNR6 | SNR6 in YEp–URA3 plasmid | Eriksson et al. (2004a) |
| pSH346 | TFIIA (TOA1 and TOA2) in YEp–LEU2 plasmid | Eriksson et al. (2004a) |
| M4793 | TFIIA (TOA1 and TOA2) in YEp–LYS2 plasmid | Biswas et al. (2004) |
| M3415 | TFIIB (SUA7) in YEp–URA3 plasmid | Mike Hampsey |
| M4480 | TBP wild-type (SPT15) in YEp–LEU2 plasmid | This work |
| M4533 | TBP wild-type (SPT15) in YEp–LYS2 plasmid | Biswas et al. (2004) |
| M4403 | TBP wild-type (SPT15) in YEp–URA3 plasmid | This work |
| pTM8 | TBP(wild-type) in YCp–TRP1 plasmid | Kobayashi et al. (2001) |
| M4471 | TBP(E93G) in YCp–TRP1 plasmid | Eriksson et al. (2004a) |
| M4325 | TBP(L114F) in YCp–TRP1 plasmid | Arndt et al. (1994) |
| M4642 | TBP(K133R) in YCp–TRP1 plasmid | Eriksson et al. (2004a) |
| M4475 | TBP(G147W) in YCp–TRP1 plasmid | Eriksson et al. (2004a) |
| M4470 | TBP(C164W) in YCp–TRP1 plasmid | Eriksson et al. (2004a) |
| M4474 | TBP(L172P) in YCp–TRP1 plasmid | Eriksson et al. (2004a) |
| M4482 | TBP(G174E) in YCp–TRP1 plasmid | Eisenmann et al. (1992) |
| M4472 | TBP(F227L) in YCp–TRP1 plasmid | Eriksson et al. (2004a) |
| M4473 | TBP(F237L) in YCp–TRP1 plasmid | Eriksson et al. (2004a) |
| M4653 | TBP(K239T) in YCp–TRP1 plasmid | Eriksson et al. (2004a) |
| M4468 | TBP(K97R, L193S) in YCp–TRP1 plasmid | Eriksson et al. (2004a) |
| M4655 | TBP(I103T, K239Stop) in YCp–TRP1 plasmid | Eriksson et al. (2004a) |
| M4550 | TBP(K133L, K145L) in YCp–TRP1 plasmid | Buratowski and Zhou (1992) |
| M4404 | TBP(K138T, Y139A) in YCp–TRP1 plasmid | Stargell and Struhl (1995) |
RNA analysis:
RNA levels were determined with S1 nuclease protection assays as described (Bhoite and Stillman 1998). The sequences of the S1 primers are as follows: CLN2, TACAACCGCCCCAAGTTTTAGCAGCCAACCAGAGACAAGTAGCGACAACCAATTTGGCTTGGTCCCGTAACACGATTCTCGGTTCC; TBP (SPT15), ACGCATGATGACAGCAGCAAAACGCTTGGGGTTATATTCTGCATTACGGGCATGTAGCGCTTGACA; TFIIB (SUA7), TCCTTGCCACTTCAGCACGTCTGCAACCAATCAGTATGGATGCAGCCATTTATGAG; TFIIA (TOA1), ATCTGCTCCTTTTCCTTGCGGGTTTTTTCCACGTCCTCCTCCTTTTCCTCGTCGTCTTTCAAGAGT; and TFIIA (TOA2), GGAGGCGTCGCGGTGGCTGTCCTCAACAGTAACCTGACAATTTTTTACGAATTTTC. The HO, CMD1, and tRNA–Trp primers have been described (Bhoite and Stillman 1998; Ozer et al. 1998).
ChIP analysis:
Chromatin immunoprecipitation was performed as described (Biswas et al. 2005), with the PCR amplifications performed in triplicate. The sequences of the PCR primers are as follows: ELP3, TGCCGCTTTCATTGTTTAATCATTTCACCTT and TCCATGACGAGCCATCTTTGTCAGGG; HXT4, TTAGTGGTGAAAAGCTTCAACACTGG and TTCAAAACCAAACCTTGATAAGAGGC; RPS5, AGGCTTAGTGGAGGTCTCACTGAA and GACTGGGGTGAATTCTTCAACAA; URA1, CCGAAGGTTATTTCACGA and CTGGCTGTCATGTTTGGT. The PCR primers for SER3 and intragenic V (used as internal control) have been described (Biswas et al. 2005).
RESULTS
Genetic interactions of Nhp6 and Gcn5 with Mot1:
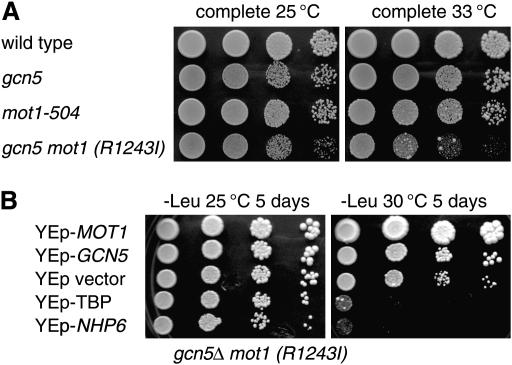
Deletion of the SPT3 gene suppresses both temperature-sensitive growth and transcriptional defects caused by the absence of Nhp6 (Yu et al. 2003). As Spt3 interacts with TBP (Eisenmann et al. 1992), we wanted to ask whether mutations in other factors that interact with TBP would suppress the nhp6ab mutation. Mot1 has also been shown to interact with TBP, and Mot1 inhibits TBP binding in vitro (Auble et al. 1994). Additionally, strong genetic links have been established for Mot1 with both Spt3 and TBP (Collart 1996; Madison and Winston 1997). MOT1 is an essential gene, but viable alleles have been identified (Abate et al. 1990; Prelich and Winston 1993; Madison and Winston 1997; Darst et al. 2003). We isolated a viable mot1 allele in a genetic screen (Jiang and Stillman 1996). We cloned this mot1 allele from the genome by allele rescue with a gapped MOT1 plasmid (Rothstein 1991) and sequenced the gene. The mutation has an arginine-to-isoleucine substitution at residue 1243. R1243 is highly conserved among Mot1 proteins, and when it is not arginine this position is usually lysine, also a basic amino acid. We crossed the mot1(R1243I) allele to a nhp6ab strain and isolated a nhp6a nhp6b mot1(R1243I) triple mutant. Instead of finding genetic suppression, we were surprised to find strong synthetic phenotypes. The nhp6ab mot1(R1243I) triple-mutant strain showed a strong growth defect at 25° and was lethal at 30° on YEPD medium (Figure 1A).
Figure 1.
Genetic interactions of MOT1 with NHP6. (A) nhp6ab mot1(R1243I) is lethal at 30°. Strains DY150 (wild type), DY7139 (nhp6ab), DY7463 [mot1(R1243I)], and DY7847 [nhp6ab mot1(R1243I)] were plated on YEPD medium for 4 days at 25° or for 2 days at 30°. (B) Strain DY7847 [nhp6ab mot1(R1243I)] was transformed with the indicated multicopy plasmids at 25°, and dilutions were plated on the indicated selective medium for 5 days at the indicated temperature.
We next asked whether multicopy plasmids could suppress the growth defect (Figure 1B). As expected, the MOT1 and NHP6A plasmids complemented, but the YEp–TFIIA and YEp–GCN5 plasmids exacerbated the growth defect at 25°. However, at 30°, YEp–SNR6 or YEp–TBP strongly suppressed the growth defect, and YEp–TFIIB showed moderate suppression. nhp6ab mutants are defective in expressing SNR6, a pol III transcribed gene encoding the U6 splicing RNA (Lopez et al. 2001; Martin et al. 2001). YEp–SNR6 suppresses the temperature-sensitive growth defect seen in nhp6ab mutants, and it is suggested that decreased SNR6 RNA contributes to the poor growth at elevated temperatures (Kruppa et al. 2001). The suppression of the nhp6ab mot1(R1243I) synthetic lethality by YEp–TBP and YEp–TFIIB suggests that this mutant strain is defective in building the TBP–TFIIB complex at promoters of pol II transcribed genes. It is less clear why overexpression of TFIIA or Gcn5 exacerbates the growth defect in the nhp6ab mot1(R1243I) strain.
We next looked for genetic interactions between GCN5 and MOT1, since Nhp6 and Gcn5 function in the same pathway for transcriptional activation of HO (Yu et al. 2000). We constructed the gcn5 mot1(R1243I) double mutant and found that it too has a strong growth defect at 25° and is nearly inviable at 30° on YEPD medium (Figure 2A). Interestingly, the growth of the nhp6a nhp6b mot1(R1243I) triple mutant at 25° is much worse than that for the gcn5 mot1(R1243I) double mutant. Figure 2B shows the effects of multicopy plasmids on growth of the gcn5 mot1(R1243I) strain. Note that while the gcn5 mot1(R1243I) strain is lethal on complete YEPD medium at 30°, it is able to grow, although poorly, on selective plates at 30°. Plasmids with MOT1 or GCN5 complemented, as expected, while multicopy plasmids with TFIIA, TFIIB, or SNR6 did not affect growth of the gcn5 mot1(R1243I) strain (data not shown). Interestingly, overexpression of TBP or Nhp6 significantly exacerbated the growth defect of the gcn5 mot1(R1243I) mutant at 30°, supporting the idea that Gcn5 and Mot1 play an active role in regulating TBP binding.
Figure 2.
Genetic interactions of MOT1 with GCN5. (A) gcn5 mot1(R1243I) is lethal at 33°. Strains DY150 (wild type), DY5265 (gcn5), DY7463 [mot1(R1243I)], and DY7841 [gcn5 mot1(R1243I)] were plated on complete medium for 3 days at either 25° or 33°. (B) Strain DY7841 [gcn5 mot1(R1243I)] was transformed with the indicated multicopy plasmids at 25°, and dilutions were plated on selective medium for 5 days at the indicated temperature.
We note that the multicopy suppression results are quite different, with YEp–TBP suppressing the nhp6ab mot1(R1243I) mutant but exacerbating the growth defect in the gcn5 mot1(R1243I) mutant. This suggests that the defects caused by the nhp6ab deletion and the mot1(R1243I) mutation are quite different.
Genetic interactions of Nhp6 and Gcn5 with the Ccr4–Not complex:
The Ccr4–Not complex has roles in regulating transcriptional initiation, elongation, and mRNA degradation (Denis and Chen 2003). A number of experiments have shown that Ccr4–Not represses transcription through direct contacts with TBP, inhibiting TBP binding to DNA (Collart 1996; Badarinarayana et al. 2000; Lemaire and Collart 2000; Deluen et al. 2002). We therefore performed genetic crosses to determine whether a ccr4 mutation might suppress nhp6ab or gcn5 defects. In the first cross we were unable to recover a viable nhp6ab ccr4 spore. To verify this apparent synthetic lethality, we constructed a nhp6aΔ/+ nhp6bΔ/+ ccr4Δ/+ triply heterozygous diploid strain and transformed it with a YCp–URA3–NHP6A plasmid. The diploids were induced to undergo meiosis, tetrads were dissected, and we isolated haploid strains with the nhp6a nhp6b ccr4 genotype containing the YCp–URA3–NHP6A plasmid. These strains were unable to grow on media containing 5-FOA (Figure 3A), indicating that the YCp–URA3–NHP6A plasmid cannot be lost. We next asked whether multicopy plasmids could suppress this synthetic lethality. For nhp6ab ccr4, the YEp–TBP plasmid partially suppressed the synthetic lethality, but YEp–TFIIA did not (Figure 3A). This is an important result, as both Ccr4 and Nhp6 have roles in transcriptional initiation and elongation, but the suppression by TBP overexpression suggests that a defect in initiation contributes to the nhp6ab ccr4 synthetic lethality. We also determined that ccr4 is synthetic lethal with a gcn5 mutation. We constructed a gcn5 ccr4 strain, containing a YCp–URA3–GCN5 plasmid, which is unable to grow on 5-FOA (Figure 3B). This synthetic lethality is partially suppressed by YEp–TFIIA, but not by YEp–TBP, YEp–TFIIB, or YEp–NHP6A (Figure 3B; data not shown).
Figure 3.
Genetic interactions of CCR4 with GCN5 and NHP6. (A) The nhp6ab ccr4 synthetic lethality is suppressed by TBP overexpression. Strain DY7441 (nhp6ab ccr4 with a YCp–URA3–NHP6B plasmid) was transformed with the indicated LYS2 multicopy plasmids, and dilutions were plated at 30° for 2 days (complete) or for 6 days (5-FOA). (B) The gcn5 ccr4 synthetic lethality is suppressed by TFIIA overexpression. Strain DY8563 [(gcn5 ccr4) with a YCp–URA3–GCN5 plasmid] was transformed with either YEp–TFIIA or the YEp–LYS2 vector, and dilutions were plated at 33° for 2 days (complete) or for 5 days (5-FOA).
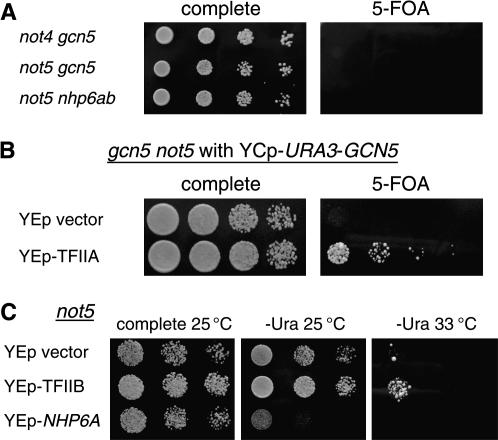
In addition to regulating TBP binding, Ccr4 is the catalytic subunit of a cytoplasmic mRNA deadenylase (Chen et al. 2002; Tucker et al. 2002). Although the Not proteins are associated with the cytoplasmic form of the Ccr4–Not complex, mutations in the NOT genes have only modest effects on the rate of deadenylation (Tucker et al. 2002), suggesting that the Not proteins and Ccr4 may have important functional differences. We therefore asked whether there are genetic interactions between nhp6ab or gcn5 and not4 and not5. For example, a haploid nhp6a nhp6b strain was crossed to a not5 mutant, and the resulting diploid was transformed with a YCp–URA3–NHP6A plasmid. After sporulation and tetrad dissection, a nhp6ab not5 triple mutant with the YCp–URA3–NHP6A plasmid was isolated. This strain was unable to grow on 5-FOA, demonstrating the synthetic lethality of nhp6ab with not5. In this way we were able to show that the nhp6ab not4, nhp6ab not5, gcn5 not4, and gcn5 not5 mutant combinations were all synthetic lethal (Figure 4A; data not shown). Multicopy suppression experiments showed that YEp–TFIIA could suppress the gcn5 not5 synthetic lethality (Figure 4B), but multicopy suppression was not seen with YEp–TFIIB, YEp–TBP, or YEp–NHP6A.
Figure 4.
Genetic interactions of NOT genes with GCN5 and NHP6. (A) A not5 mutation is synthetic lethal with gcn5 and with nhp6, and not4 is synthetic lethal with gcn5. Dilutions of strains DY8618 (not4 gcn5), DY8628 (not5 gcn5), and DY8625 (not5 nhp6ab), each carrying a YCp–URA3 plasmid with either GCN5 or NHP6A, were plated on the indicated medium at 25° for 3 days. (B) The gcn5 not5 synthetic lethality is suppressed by TFIIA overexpression. Strain DY8628 [(gcn5 not5) with a YCp–URA3–GCN5 plasmid] was transformed with either YEp–TFIIA or the YEp–LYS2 vector and plated at 25° for 2 days on complete medium or for 5 days on 5-FOA plates. (C) Growth of the not5 mutant is affected by TFIIB or Nhp6 overexpression. Strain DY8626 (not5) was transformed with the indicated URA3 multicopy plasmids, and dilutions were plated for 4 days (complete at 25°), 2 days (−Ura at 25°), or 6 days (−Ura at 33°).
We observed synthetic lethality of gcn5 with all three members of the Ccr4–Not complex that we tested: ccr4, not4, and not5. In contrast, Maillet et al. (2000) did not observe synthetic lethality in gcn5 ccr4 or gcn5 not5 mutants and saw only a synthetic slow-growth defect in the gcn5 not4 double mutant. We used W303 strains, while their studies utilized a different strain background, and strain differences could be responsible for the different results.
The not5 single mutant shows a growth defect at 30° and is unable to grow at the higher temperature of 33°. Thus, we asked whether overexpression of other factors affected growth of the not5 mutant. The not5 strain was transformed with multicopy plasmids and then growth at various temperatures was assessed. While multicopy plasmids with TBP or TFIIA did not affect growth of the not5 mutant, YEp–TFIIB improved growth at 25° and partially suppressed the temperature-sensitive growth defect (Figure 4). In contrast, overexpression of Nhp6 exacerbated the not5 growth defect, even at 25° (Figure 4; data not shown). This exacerbation of the not5 growth defect by the multicopy plasmid with NHP6A reinforces the role of Nhp6 in RNA pol II transcription.
spt3 is synthetic lethal with mot1 or ccr4:
Spt3 physically interacts with TBP, and Spt3 acts to either promote or inhibit TBP binding, depending on the promoter (Eisenmann et al. 1992; Dudley et al. 1999; Belotserkovskaya et al. 2000; Bhaumik and Green 2002; Barbaric et al. 2003; Yu et al. 2003). Additionally, we have observed that an spt3 mutation can suppress growth defects in both nhp6a nhp6b and gcn5 nhp6a nhp6b strains (Yu et al. 2003) and the synthetic lethality of TBP mutants in gcn5 or nhp6ab strains (Biswas et al. 2004; Eriksson et al. 2004a). On the basis of these results, we tested whether an spt3 gene disruption can suppress the synthetic lethality of a mot1 mutation with gcn5 or nhp6ab. A mot1(R1243I) mutant was crossed to a gcn5 spt3 strain, and we found that spt3 mot1(R1243I) double mutants are synthetic lethal, consistent with an earlier report using a different mot1 allele (Madison and Winston 1997). We also crossed the mot1(R1243I) mutant to a nhp6ab spt3 strain but we were unable to isolate a mot1(R1243I) nhp6ab spt3 strain. Thus spt3 cannot suppress these synthetic lethalities with mot1.
We next asked whether spt3 could suppress the synthetic lethality of a ccr4 mutation with either gcn5 or nhp6ab. In these crosses we did not recover any viable ccr4 spt3 strains, irrespective of the GCN5 or NHP6 genotype, suggesting that ccr4 and spt3 are synthetically lethal. To test this idea, we transformed a +/ccr4 +/spt3 heterozygous diploid strain with a YCp–URA3 plasmid with either CCR4 or SPT3, and haploid ccr4 spt3 segregants with either YCp–URA3–CCR4 or YCp–URA3–SPT3 were isolated. These haploid strains were unable to grow on 5-FOA, demonstrating the ccr4 spt3 synthetic lethality. This result disagrees with that of Badarinarayana et al. (2000), who found the ccr4 spt3 double mutant viable in their strain background.
Synthetic lethality of TBP mutants with mot1 and ccr4:
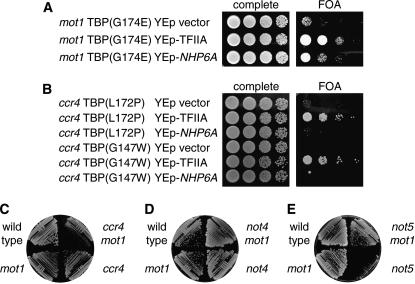
We recently conducted a screen to identify TBP mutants that are viable, but lethal in the absence of Nhp6 (Eriksson et al. 2004a). Many of these TBP mutants are also lethal in a gcn5 mutant (Biswas et al. 2004), and we decided to test whether mot1 or ccr4 mutations affected viability of these TBP mutants. We constructed a mot1(R1243I) spt15Δ double mutant, kept alive by the wild-type SPT15 (TBP) gene on a YCp–URA3 plasmid. This strain was transformed with 14 TBP mutants on YCp–TRP1 plasmids, and we used plasmid shuffling to assess the viability of the mot1(R1243I) spt15 strains on 5-FOA media at 25°, 30°, and 35°, where the YCp–URA3—TBP (wild-type) plasmid must be lost for cells to grow (Table 3). Three TBP mutants were synthetic lethal with mot1(R1243I) at all temperatures tested, and 9 others either were synthetic lethal or showed very poor growth at 35°. All of these TBP mutants grew well at 35° in a MOT1 strain (data not shown). None of these mot1(R1243I) TBP synthetic interactions could be suppressed by a multicopy plasmid with NHP6A (Table 3). However, the synthetic lethality at 35° between mot1(R1243I) and the G174E substitution in TBP [spt15(G174E)] could be suppressed by overexpression of TFIIA (Figure 5A ). Two conclusions result from these genetic experiments. First, most of these TBP mutants show a major growth defect when combined with mot1(R1243I). Second, overexpression of TFIIA can suppress the mot1(R1243I) spt15(G174E) lethality, suggesting that Mot1 may contribute to formation of the TBP–TFIIA–DNA complex.
TABLE 3.
Synthetic lethality of TBP mutants with mot1 and ccr4
|
spt15 (TBP) mutant
|
Phenotype in mot1(R1243I)
|
Suppression of mot1(R1243I) spt15 by:
|
Phenotype in ccr4
|
Suppression of ccr4 spt15 by:
|
||
|---|---|---|---|---|---|---|
| YEp–TFIIA | YEp–NHPA | YEp–TFIIA | YEp–NHPA | |||
| E93G | S.L. 35° | No effect | No effect | S.L. | Supp. | No effect |
| L114F | S.L. | No effect | No effect | S.L. | No effect | No effect |
| K133R | S.L. | No effect | No effect | Viable | ||
| G147W | Viable | S.L. | Supp. | No effect | ||
| C164W | Poor growth 35° | No effect | No effect | Viable | ||
| L172P | S.L. 35° | No effect | No effect | Poor growth | Supp. | Supp. |
| G174E | S.L. 35° | Supp. | No effect | Poor growth | ND | ND |
| F227L | S.L. 35° | No effect | No effect | Viable | ||
| F237L | S.L. 35° | No effect | No effect | Viable | ||
| K239T | S.L. 35° | No effect | No effect | Viable | ||
| K97R, L193S | S.L. 35° | No effect | No effect | Poor growth | ND | ND |
| I103T, K239Stop | S.L. 35° | No effect | No effect | Poor growth | ND | ND |
| K133L,K145L | Viable | Viable | ||||
| K138T,Y139A | S.L. | No effect | No effect | S.L. | No effect | No effect |
S.L., synthetic lethal at all temperatures; S.L. 35°, viable at 25° and 30°, but lethal at 35°; Supp., suppression; ND, not determined.
Figure 5.
Suppression of mot1 spt15 and ccr4 spt15 synthetic lethality. (A) The mot1(R1243I) spt15(G174E) [TBP(G174E)] synthetic lethality is suppressed by TFIIA or Nhp6 overexpression. Strain DY9383 [mot1(R1243I) spt15Δ with a YCp–URA3–SPT15(wild-type) plasmid] was transformed with the YCp–TRP1–TBP(G174E) plasmid and the indicated LYS2 multicopy plasmids and grown for 3 days on complete medium at 25° or on 5-FOA medium at 34°. (B) The ccr4 spt15 synthetic lethalities are suppressed by TFIIA overexpression. Strain DY9384 [ccr4 spt15Δ with a YCp–URA3–SPT15(wild-type) plasmid] was transformed with either the YCp–TRP1–TBP(G147W) or the YCp–TRP1–TBP(L172P) plasmid and the indicated LYS2 multicopy plasmids and grown at 35° on complete medium for 2 days or on 5-FOA medium for 3 days. (C) Synthetic growth defect in the ccr4 mot1 double mutant. Strains DY150 (wild type), DY7462 [mot1(R1243I)], DY7176 (ccr4), and DY9470 [ccr4 mot1(R1243I)] were grown on complete medium at 30° for 2 days. (D) No additive effect in the not4 mot1 double mutant. Strains DY150 (wild type), DY7462 [mot1(R1243I)], DY8617 (not4), and DY9545 [not4 mot1(R1243I)] were grown on complete medium at 30° for 4 days. (E) mot1 suppresses the not5 growth defect. Strains DY150 (wild type), DY7462 [mot1(R1243I)], DY8627 (not5), and DY9582 [not5 mot1(R1243I)] were grown on complete medium at 30° for 3 days.
We next constructed a ccr4 spt15 double-deletion mutant with the wild-type SPT15 (TBP) gene on a YCp–URA3 plasmid. This strain was transformed with the same 14 TBP mutants and the ability of these transformants to grow at various temperatures on 5-FOA without the wild-type TBP gene was assessed (Table 3). Four TBP mutants were synthetic lethal at all temperatures in the ccr4 mutant, and four other TBP mutants showed poor growth at all temperatures in the ccr4 mutant. To assess multicopy suppression, the ccr4 spt15 YCp–URA3 TBP (wild-type) strain was transformed with the TBP mutants and YEp–TFIIA, YEp–NHP6A, or the YEp vector control. In several instances, overexpression of TFIIA or NHP6A suppressed the synthetic growth defects (Table 3; Figure 5B). For example, the ccr4 spt15(G147W) synthetic lethality is suppressed by YEp–TFIIA, and the ccr4 spt15(L172P) growth defect is suppressed by overexpression of either TFIIA or NHP6A. The synthetic lethality between ccr4 and TBP mutants, along with suppression by overexpression of TFIIA, strongly supports a role for Ccr4 either in facilitating the interaction between TBP and TFIIA or in TBP binding at promoters.
Interestingly, the pattern of synthetic lethality is different for mot1(R1243I) and ccr4. For example, the K133R substitution in TBP [spt15(K133R)] is lethal in mot1(R1243I) but viable in ccr4, while spt15(G147W) shows an opposite pattern. This result suggests that Mot1 and Ccr4/Not have nonidentical roles in regulating TBP. To test this idea, we crossed a mot1(R1243I) mutant to three strains with mutations in CCR4, NOT4, or NOT5 and examined the growth of double-mutant strains. The ccr4 mot1(R1243I) double mutant shows a growth defect, compared to either single mutant (Figure 5C), and the not4 mot1(R1243I) double mutant shows no additive effect (Figure 5D). The results with the not5 mot1(R1243I) double mutant (Figure 5E) are quite striking. The not5 mutant is essentially unable to grow at 30°, but this growth defect is completely suppressed in the not5 mot1(R1243I) double mutant. This suppression strongly argues that Mot1 and Ccr4/Not have quite different roles in transcriptional regulation.
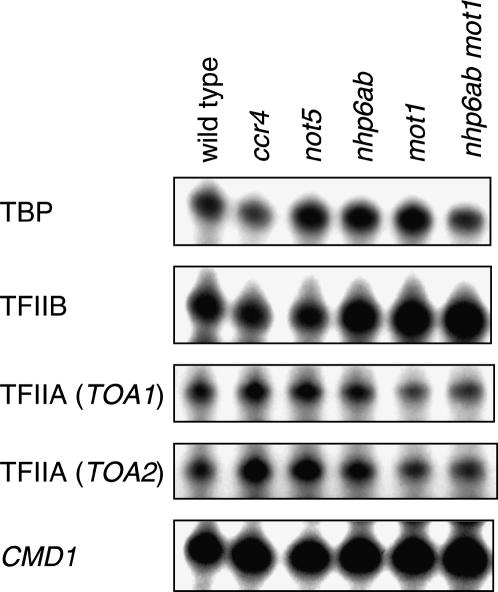
Overexpression of basal transcription factors suppresses some genetic defects involving nhp6, gcn5, mot1(R1243I), ccr4, and not5 (Figures 1–4). One explanation for these results is that expression of basal factors is reduced in these mutants, and thus overexpression suppresses growth defects. To address this question, we determined mRNA levels for TBP (SPT15 mRNA), TFIIB (SUA7 mRNA), and TFIIA (two subunits, TOA1 and TOA2 mRNA). The results in Figure 6 show that these mutations in nhp6, gcn5, mot1(R1243I), ccr4, and not5 do not significantly affect mRNA levels for basal transcription factors.
Figure 6.
Basal factor expression is not affected by mutants. RNA was prepared from strains DY150 (wild type), DY7176 (ccr4), DY8626 (not5), DY7139 (nhp6a), DY7463 [mot1(R1243I)], and DY7847 [nhp6ab mot1(R1243I)] grown at 25° and used for S1 nuclease protection assays to measure TBP (SPT15), TFIIB (SUA7), and TFIIA subunits one (TOA1) and two (TOA2) and CMD1 (internal control) RNA levels.
Additive effects on HO expression in double mutants:
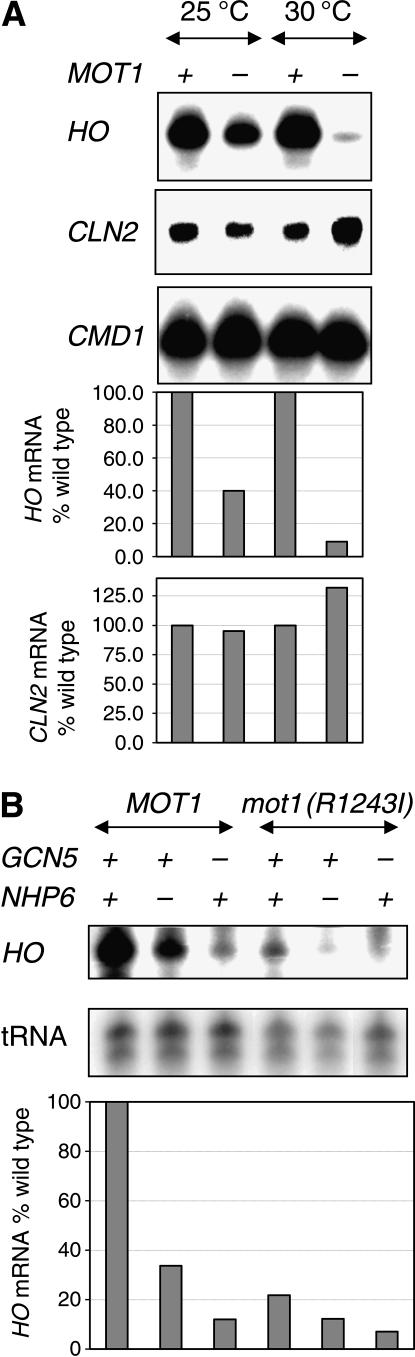
As both Gcn5 and Nhp6 are required for full activation of the HO gene (Yu et al. 2003), we determined whether a mot1(R1243I) mutation affected HO expression. HO mRNA levels are reduced to ∼40% of wild type in the mot1(R1243I) strain grown at 25° and reduced to 9% when grown at 30° (Figure 7A). HO is cell cycle regulated, and thus a defect in cell cycle progression could reduce the fraction of cells in late G1, when HO is expressed. To address this question, we also measured CLN2 mRNA levels; CLN2 is expressed in late G1, coincident with HO. The mot1(R1243I) mutation does not affect CLN2 levels, and thus an alteration in the cell cycle does not cause the decreased HO expression. In contrast to mot1(R1243I), a ccr4 mutation does not affect HO expression (data not shown).
Figure 7.
HO expression is reduced in mutant strains. (A) RNA was prepared from strains DY150 (wild type) and DY7462 [mot1(R1243I)] grown at either 25° or 30° and used for S1 nuclease protection assays to measure HO, CLN2, and CMD1 (internal control) RNA levels. (B) RNA was prepared from strains DY150 (wild type), DY7463 [mot1(R1243I)], DY5265 (gcn5), DY7841 [gcn5 mot1(R1243I)], DY7139 (nhp6ab), and DY7847 [nhp6ab mot1(R1243I)] grown at 25° and used for S1 nuclease protection assays to measure HO and tRNA–Trp (internal control) RNA levels.
On the basis of the additive growth defect in gcn5 mot1(R1243I) and nhp6a nhp6b mot1(R1243I) mutants, we looked for additive effects in transcriptional activation at HO. Cells were grown at 25°, as some of the strains have severe growth defects at higher temperatures, and RNA was isolated for S1 nuclease protection assays. As shown previously, HO expression is reduced in the nhp6ab and gcn5 strains (Figure 7B). Interestingly, there is an additive decrease in HO mRNA levels in the nhp6ab mot1(R1243I) triple mutant, compared to the nhp6ab and mot1(R1243I) strains. There is a similar additive effect in the gcn5 mot1(R1243I) double mutant compared to the corresponding single mutants. These results are consistent with the idea that Nhp6, Gcn5, and Mot1 function through distinct mechanisms, although the effects may be on a common target. We have previously shown that the defect in HO expression in nhp6ab and gcn5 mutants can be suppressed by overexpression of TBP (Yu et al. 2003).
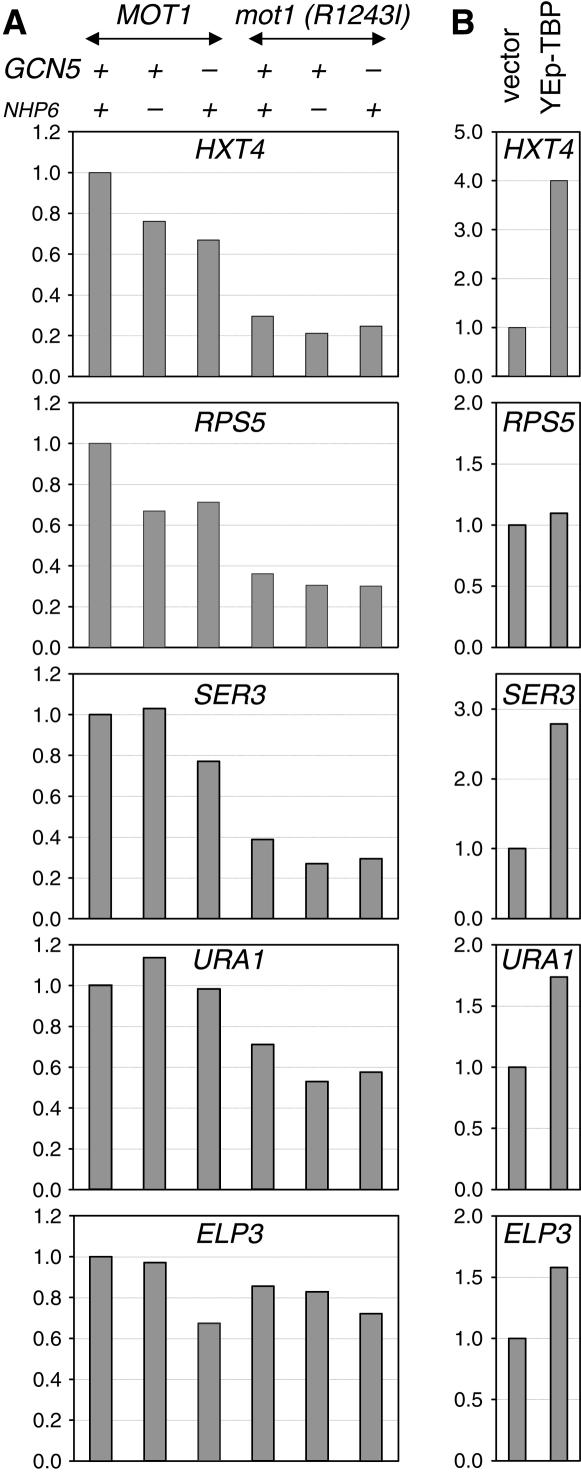
Effects of mutations on TBP binding at promoters:
We used ChIP assays to measure TBP binding to promoters in mutants. Cells were grown at 25°, shifted to 37° for 3 hr, and then treated with formaldehyde for crosslinking. After immunoprecipitation with anti-TBP antibody and reversal of crosslinks, TBP binding to various promoters was measured by real time PCR. As shown in Figure 8A, there is decreased TBP binding to the RPS5, HXT4, SER3, and URA1 promoters in the nhp6, gcn5, and mot1(R1243I) mutants. Other mot1 mutations have previously been shown to affect TBP binding to HXT4 and URA1 (Dasgupta et al. 2005; van Oevelen et al. 2005). Importantly, not all promoters are affected so strongly, for example, ELP3. When we look at the multiply mutant strains, such as nhp6ab mot1(R1243I) and gcn5 mot1(R1243I), there are additive defects in TBP binding, although the additivity is modest. Overexpression of TBP suppresses the growth defect of nhp6ab mot1(R1243I) cells. We therefore examined TBP binding in nhp6ab mot1(R1243I) cells with the YEp–TBP plasmid (Figure 8B); the control for this experiment is the same strain with the YEp vector without an insert. TBP overexpression results in a significant increase in TBP binding at several promoters in these cells. These results support the idea that a defect in TBP binding to promoters contributes to the growth defect seen in these multiply mutant strains.
Figure 8.
Mutations affect TBP binding to promoters. TBP occupancy at the indicated promoters was determined by chromatin immunoprecipitation with polyclonal anti-TBP antisera and quantitative PCR, using cells that had been grown at 25° and then shifted to 37° for 3 hr. Relative binding is shown, after normalization to an intergenic V internal control. The average of replicate PCR amplifications is shown. (A) TBP binding is reduced in mutants. Strains DY150 (wild type), DY7463 [mot1(R1243I)], DY5265 (gcn5), DY7841 [gcn5 mot1(R1243I)], DY7139 (nhp6ab), and DY7847 [nhp6ab mot1(R1243I)] were grown on YEPD media. (B) Multicopy TBP plasmid restores TBP binding in the nhp6ab mot1(R1243I) strain. DY7847 [nhp6ab mot1(R1243I)] with either the YEp vector control or a YEp plasmid with the gene encoding TBP were grown on selective medium.
DISCUSSION
We have previously shown that the Nhp6 architectural transcription factor and the Gcn5 histone acetyltransferase function in parallel pathways in activation of the yeast HO gene (Yu et al. 2000), and our data suggest that both Nhp6 and Gcn5 could affect DNA binding by TBP (Biswas et al. 2004; Eriksson et al. 2004a). To further explore the roles of these factors, in this report we have examined the effect of combining nhp6ab or gcn5 gene disruptions with mutations affecting known regulators of DNA binding by TBP. Both biochemical and genetic experiments show Mot1 regulates TBP binding to DNA, and the mot1(R1243I) allele is lethal when combined with either nhp6ab or gcn5. The Ccr4–Not complex has multiple roles in gene regulation, and genetic experiments suggest one role in regulating TBP binding. We tested gene disruptions affecting three members of the Ccr4–Not complex, ccr4, not4, and not5, and all three were synthetically lethal when combined with either nhp6ab or gcn5. We have recently isolated point mutations in TBP that are viable in wild-type strains but lethal in nhp6ab or gcn5 mutants (Biswas et al. 2004; Eriksson et al. 2004a). We have tested 14 of these TBP mutants in mot1 or ccr4 mutants, and most of them show synthetic growth defects or lethality when combined with mot1 or ccr4. A not5 mutation has a severe growth defect at 30°, but this is suppressed by a mot1 mutation. Interestingly, many of the synthetic lethal phenotypes described in this report can be suppressed by overexpression of TFIIA, suggesting that these various regulators all work to stimulate either TBP binding or the interaction of TBP and TFIIA with DNA.
In vitro studies show that the Mot1 protein is able to remove TBP from binding sites, in an ATP-dependent fashion (Auble et al. 1994; Darst et al. 2003), and that in vivo Mot1 protein is present in a complex with TBP (Poon et al. 1994). Chromatin immunoprecipitation experiments show that Mot1 associates with promoters (Andrau et al. 2002; Dasgupta et al. 2002) and that a mot1 mutation affects TBP binding to promoters in vivo (Li et al. 1999; Geisberg et al. 2002). Mot1 co-occupies promoters with TBP, but not with TFIIB, TFIIA, or pol II under normal conditions, suggesting that Mot1 functions as a repressor (Geisberg and Struhl 2004). Expression profiling studies show that mot1 mutations reduce expression of some genes and derepress others (Andrau et al. 2002; Dasgupta et al. 2002; Geisberg et al. 2002), arguing that Mot1 functions as either an activator or a repressor at different promoters. However, Geisberg and Struhl (2004) show that when cells are heat-shocked or stressed Mot1 does co-occupy promoters with TFIIB and RNA pol II, suggesting that these preinitiation complexes contain Mot1. They suggest the stress response resulting from thermal inactivation of mutant Mot1 indirectly causes decreased expression of some genes in the microarray studies. It is intriguing that under stress conditions Mot1 and TFIIA do not co-occupy promoters, suggesting that these preinitiation complexes contain Mot1 instead of TFIIA (Geisberg and Struhl 2004). Interestingly, there are data suggesting that Mot1 and TFIIA have opposing effects both in vivo and in vitro (Auble and Hahn 1993; Madison and Winston 1997; Chicca et al. 1998). Finally, Dasgupta et al. (2005) recently showed that TBP is bound to Mot1-activated genes following Mot1 inactivation, but other basal factors are not bound. This results suggests Mot1 mediates repression by displacing TBP from chromatin.
There are several ways to explain the observed mot1 nhp6ab and mot1 gcn5 synthetic lethalities. One explanation is that full Mot1 activity is required for efficient expression of specific genes during stress response, and either the nhp6ab or gcn5 mutations reduce expression of these genes. However, expression profiles of nhp6ab and gcn5 mutants do not show decreased expression of stress response genes (Lee et al. 2000; Moreira and Holmberg 2000; our unpublished observations). We favor another explanation where Mot1, Nhp6, and Gcn5 all function in the same pathway, that of affecting TBP binding to DNA at some genes. In support of this hypothesis, we note that the mot1 nhp6ab synthetic lethality is suppressed by TBP overexpression (Figure 1) and that the mot1 gcn5 defect is much worse when either TBP or Nhp6 is overexpressed (Figure 2). Additionally, the lethality resulting from combining TBP point mutations with either mot1 or gcn5 can be suppressed by overexpression of TFIIA (Figure 5) (Biswas et al. 2004). ChIP experiments show that nhp6ab, gcn5, and mot1 mutations all lead to reduced TBP binding to promoters (Figure 8).
Genetic and biochemical studies suggest that the Ccr4–Not complex is a regulator of TBP binding, along with roles in transcriptional elongation and mRNA degradation. Mutations in different genes encoding subunits of Ccr4–Not have different phenotypes, suggesting that different subunits make contributions toward different functions (Collart 2003). For example, Ccr4 is part of the cytoplasmic mRNA deadenylase (Tucker et al. 2001), and while ccr4 mutations have a major impact on deadenylation activity, not mutations have small effects on deadenylation (Tucker et al. 2002). Additionally, the Ccr4 protein, but not other members of the Ccr4–Not complex, is associated with the Paf1 complex that travels with elongating RNA polymerase (Chang et al. 1999). A ccr4 paf1 double mutant is lethal, but combining any of the not mutations with paf1 is viable (Chang et al. 1999; Maillet et al. 2000). Additionally, Not4 has been recently shown to be a ubiquitin ligase (Albert et al. 2002), although further work is needed to identify the targets of ubiquitylation and to determine how ubiquitylation affects transcriptional regulation.
The not mutations were isolated as global repressors that affected TBP binding at TATA-less promoters (Collart and Struhl 1994). The Not1, Not2, and Not5 proteins physically interact with TBP or TAFs, the TBP-associated factors present in TFIID (Badarinarayana et al. 2000; Lemaire and Collart 2000; Sanders et al. 2002), and not4 and not5 mutations show synthetic lethality in combination with taf mutations (Lemaire and Collart 2000). Additionally, not4 mutations suppress the transcriptional defect caused by Ty insertions into the HIS4 promoter (Badarinarayana et al. 2000), a phenotype also seen in spt15 (TBP), spt3, and mot1 mutants (Jiang and Stillman 1996; Madison and Winston 1997; Winston and Sudarsanam 1998). Mutations in genes encoding the Ccr4–Not complex affect binding of TBP and TAF1 to promoters (Lenssen et al. 2005).
Thus the evidence linking the NOT genes to regulation of TBP is quite strong. Our genetic data bring Nhp6 and Gcn5 into the same pathway as the Ccr4–Not complex in regulating TBP binding. We believe that the synthetic lethality caused by combining a ccr4, not4, or a not5 mutation with either gcn5 or nhp6ab results from a dysregulation of TBP binding. The fact that overexpression of TBP or TFIIA can suppress some of these synthetic lethalities supports this idea.
Both the Mot1 and the Ccr4/Not complex regulate TBP binding, but it is not clear whether they do so in the same or different pathways. We find that the ccr4 mot1(R1243I) double mutant shows a growth defect, and more significantly, mot1(R1243I) suppresses not5 growth defects. The not5 mutant is unable to grow at 30°, but the not5 mot1(R1243I) double mutant does grow (Figure 5E). This suggests that the not5 mutant is defective in some aspect of transcriptional activation and that the mot1(R1243I) allele has properties that overcome this defect. We also note that the not5 growth defect can be partially suppressed by overexpression of TFIIB (Figure 4C). We suggest that the Mot1 and Ccr4/Not complexes function in distinct pathways in regulating TBP.
The Spt3 component of the SAGA complex interacts both physically and genetically with TBP (Eisenmann et al. 1992). Spt3 is required for TBP recruitment to the GAL1 and PHO5 promoters in vivo (Dudley et al. 1999; Barbaric et al. 2003), but Spt3 inhibits TBP binding to the HO promoter (Yu et al. 2003). spt3 and mot1 are synthetically lethal, and this synthetic lethality can be suppressed by overexpression of TFIIA (Madison and Winston 1997). Interestingly, both spt3 and mot1 are synthetic lethal with substitutions in the Toa1 subunit of TFIIA (Madison and Winston 1997). Both Spt3 and Mot1 are required for nucleosome remodeling at Gal4-dependent promoters (Topalidou et al. 2004). Moreover, Spt3 is required for Mot1 to bind to the GAL1 promoter under inducing conditions, and Mot1 is similarly required for Spt3 binding (Topalidou et al. 2004). We note a number of synthetic lethalities or growth defects among these genes: mot1 spt3 (Madison and Winston 1997), ccr4 spt3, and ccr4 mot1. We attribute these additive genetic defects to a common target, TBP.
mot1 mutations reduce TBP binding to certain promoters (Andrau et al. 2002), while TBP binding to the INO1 promoter was unaffected by a mot1 mutation (Dasgupta et al. 2005). We chose to study TBP binding in strains with a mot1 mutation alone or in combination with gcn5 and nhp6ab mutations. Our results show that TBP binding at selected promoters is significantly reduced in a mot1 mutant (Figure 8). TBP binding is further reduced, although modestly, when mot1 is combined with either gcn5 or nhp6ab. The mot1 nhp6ab strain shows reduced binding of basal transcription factors, and suppression of this defect by overexpression of TBP further supports our hypothesis that the mot1 and nhp6ab mutations cause defects in TBP binding. RNA analysis shows that HO expression is reduced in a mot1 strain and is further reduced when combined with other mutations such as gcn5 or nhp6ab (Figure 7).
An spt3 gene deletion suppresses several nhp6ab defects, including reduced HO expression, temperature-sensitive growth, and synthetic lethality with TBP mutants (Yu et al. 2003; Eriksson et al. 2004a). spt3 also suppresses the synthetic lethality resulting from combining gcn5 with nhp6ab and the reduced HO expression in a gcn5 mutant. Additionally, either a spt3 mutation or a TBP mutation that disrupts the TBP–Spt3 interaction can suppress the temperature sensitivity of not1-2 (Collart 1996).
The genetic analyses involving TBP, TFIIA, Nhp6, Gcn5, Mot1, Ccr4–Not, and Spt3 show both synthetic lethality and genetic suppression. Taken together, these genetic interactions strongly support a role for these factors in regulating DNA binding of TBP and TFIIA. Further work, particularly at the biochemical level, will be needed to understand exactly how these factors regulate TBP–TFIIA binding to promoters.
Acknowledgments
We thank David Auble, Martine Collart, Clyde Denis, Steve Hahn, Mike Hampsey, and Tony Weil, who provided plasmids, strains, or antibodies. We also acknowledge Yiwei Jiang who constructed plasmid M2719. We thank David Auble and Clyde Denis for comments on the manuscript. This work was supported by a grant from the National Institutes of Health awarded to D.J.S.
References
- Abate, C., D. Luk, R. Gentz, F. J. Rauscher, III and T. Curran, 1990. Expression and purification of the leucine zipper and DNA-binding domains of Fos and Jun: both Fos and Jun contact DNA directly. Proc. Natl. Acad. Sci. USA 87: 1032–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert, T. K., H. Hanzawa, Y. I. Legtenberg, M. J. de Ruwe, F. A. van den Heuvel et al., 2002. Identification of a ubiquitin-protein ligase subunit within the CCR4-NOT transcription repressor complex. EMBO J. 21: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrau, J. C., C. J. van Oevelen, H. A. van Teeffelen, P. A. Weil, F. C. Holstege et al., 2002. Mot1p is essential for TBP recruitment to selected promoters during in vivo gene activation. EMBO J. 21: 5173–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt, K. M., C. R. Wobbe, S. Ricupero-Hovasse, K. Struhl and F. Winston, 1994. Equivalent mutations in the two repeats of yeast TATA-binding protein confer distinct TATA recognition specificities. Mol. Cell. Biol. 14: 3719–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auble, D. T., and S. Hahn, 1993. An ATP-dependent inhibitor of TBP binding to DNA. Genes Dev. 7: 844–856. [DOI] [PubMed] [Google Scholar]
- Auble, D. T., K. E. Hansen, C. G. Mueller, W. S. Lane, J. Thorner et al., 1994. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 8: 1920–1934. [DOI] [PubMed] [Google Scholar]
- Badarinarayana, V., Y. C. Chiang and C. L. Denis, 2000. Functional interaction of CCR4-NOT proteins with TATAA-binding protein (TBP) and its associated factors in yeast. Genetics 155: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y., G. M. Perez, J. M. Beechem and P. A. Weil, 1997. Structure-function analysis of TAF130: identification and characterization of a high-affinity TATA-binding protein interaction domain in the N terminus of yeast TAF(II)130. Mol. Cell. Biol. 17: 3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaric, S., H. Reinke and W. Horz, 2003. Multiple mechanistically distinct functions of SAGA at the PHO5 promoter. Mol. Cell. Biol. 23: 3468–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belotserkovskaya, R., D. E. Sterner, M. Deng, M. H. Sayre, P. M. Lieberman et al., 2000. Inhibition of TATA-binding protein function by SAGA subunits Spt3 and Spt8 at Gcn4-activated promoters. Mol. Cell. Biol. 20: 634–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik, S. R., and M. R. Green, 2002. Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo. Mol. Cell. Biol. 22: 7365–7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoite, L. T., and D. J. Stillman, 1998. Residues in the Swi5 zinc finger protein that mediate cooperative DNA-binding with the Pho2 homeodomain protein. Mol. Cell. Biol. 18: 6436–6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas, D., A. N. Imbalzano, P. Eriksson, Y. Yu and D. J. Stillman, 2004. Role for Nhp6, Gcn5, and the Swi/Snf complex in stimulating formation of the TATA-binding protein-TFIIA-DNA complex. Mol. Cell. Biol. 24: 8312–8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas, D., Y. Yu, M. Prall, T. Formosa and D. J. Stillman, 2005. The yeast FACT complex has a role in transcriptional initiation. Mol. Cell. Biol. 25: 5812–5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke, J. D., F. LaCroute and G. R. Fink, 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197: 345–346. [DOI] [PubMed] [Google Scholar]
- Buratowski, S., and H. Zhou, 1992. Transcription factor IID mutants defective for interaction with transcription factor IIA. Science 255: 1130–1132. [DOI] [PubMed] [Google Scholar]
- Chang, M., D. French-Cornay, H. Y. Fan, H. Klein, C. L. Denis et al., 1999. A complex containing RNA polymerase II, Paf1p, Cdc73p, Hpr1p, and Ccr4p plays a role in protein kinase C signaling. Mol. Cell. Biol. 19: 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Y. C. Chiang and C. L. Denis, 2002. CCR4, a 3′-5′ poly(A) RNA and ssDNA exonuclease, is the catalytic component of the cytoplasmic deadenylase. EMBO J. 21: 1414–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicca, J. J., III, D. T. Auble and B. F. Pugh, 1998. Cloning and biochemical characterization of TAF-172, a human homolog of yeast Mot1. Mol. Cell. Biol. 18: 1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero and P. Hieter, 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110: 119–122. [DOI] [PubMed] [Google Scholar]
- Coleman, R. A., and B. F. Pugh, 1997. Slow dimer dissociation of the TATA binding protein dictates the kinetics of DNA binding. Proc. Natl. Acad. Sci. USA 94: 7221–7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart, M. A., 1996. The NOT, SPT3, and MOT1 genes functionally interact to regulate transcription at core promoters. Mol. Cell. Biol. 16: 6668–6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart, M. A., 2003. Global control of gene expression in yeast by the Ccr4-Not complex. Gene 313: 1–16. [DOI] [PubMed] [Google Scholar]
- Collart, M. A., and K. Struhl, 1994. NOT1/CDC39, NOT2/CDC36, NOT3 and NOT4 encode a global-negative regulator of transcription that differentially affects TATA-element utilization. Genes Dev. 8: 525–537. [DOI] [PubMed] [Google Scholar]
- Costigan, C., D. Kolodrubetz and M. Snyder, 1994. NHP6A and NHP6B, which encode HMG1-like proteins, are candidates for downstream components of the yeast SLT2 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 14: 2391–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darst, R. P., A. Dasgupta, C. Zhu, J. Y. Hsu, A. Vroom et al., 2003. Mot1 regulates the DNA binding activity of free TATA-binding protein in an ATP-dependent manner. J. Biol. Chem. 278: 13216–13226. [DOI] [PubMed] [Google Scholar]
- Dasgupta, A., R. P. Darst, K. J. Martin, C. A. Afshari and D. T. Auble, 2002. Mot1 activates and represses transcription by direct, ATPase-dependent mechanisms. Proc. Natl. Acad. Sci. USA 99: 2666–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta, A., S. A. Juedes, R. O. Sprouse and D. T. Auble, 2005. Mot1-mediated control of transcription complex assembly and activity. EMBO J. 24: 1717–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluen, C., N. James, L. Maillet, M. Molinete, G. Theiler et al., 2002. The Ccr4-Not complex and yTAF1 (yTaf(II)130p/yTaf(II)145p) show physical and functional interactions. Mol. Cell. Biol. 22: 6735–6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis, C. L., and J. Chen, 2003. The CCR4-NOT complex plays diverse roles in mRNA metabolism. Prog. Nucleic Acid Res. Mol. Biol. 73: 221–250. [DOI] [PubMed] [Google Scholar]
- Dudley, A. M., C. Rougeulle and F. Winston, 1999. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 13: 2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir, A., J. W. Conaway and R. C. Conaway, 2001. Mechanism of transcription initiation and promoter escape by RNA polymerase II. Curr. Opin. Genet. Dev. 11: 209–214. [DOI] [PubMed] [Google Scholar]
- Eisenmann, D. M., K. M. Arndt, S. L. Ricupero, J. W. Rooney and F. Winston, 1992. SPT3 interacts with TFIID to allow normal transcription in Saccharomyces cerevisiae. Genes Dev. 6: 1319–1331. [DOI] [PubMed] [Google Scholar]
- Eriksson, P., D. Biswas, Y. Yu, J. M. Stewart and D. J. Stillman, 2004. a TATA-binding protein mutants that are lethal in the absence of the Nhp6 high-mobility-group protein. Mol. Cell. Biol. 24: 6419–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, P., L. R. Thomas, A. Thorburn and D. J. Stillman, 2004. b pRS yeast vectors with a LYS2 marker. BioTechniques 36: 212–213. [DOI] [PubMed] [Google Scholar]
- Formosa, T., P. Eriksson, J. Wittmeyer, J. Ginn, Y. Yu et al., 2001. Spt16-Pob3 and the HMG protein Nhp6 combine to form the nucleosome- binding factor SPN. EMBO J. 20: 3506–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragiadakis, G. S., D. Tzamarias and D. Alexandraki, 2004. Nhp6 facilitates Aft1 binding and Ssn6 recruitment, both essential for FRE2 transcriptional activation. EMBO J. 23: 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisberg, J. V., and K. Struhl, 2004. Cellular stress alters the transcriptional properties of promoter-bound Mot1-TBP complexes. Mol. Cell 14: 479–489. [DOI] [PubMed] [Google Scholar]
- Geisberg, J. V., Z. Moqtaderi, L. Kuras and K. Struhl, 2002. Mot1 associates with transcriptionally active promoters and inhibits association of NC2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 22: 8122–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, R. D., and A. Sugino, 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74: 527–534. [DOI] [PubMed] [Google Scholar]
- Goppelt, A., G. Stelzer, F. Lottspeich and M. Meisterernst, 1996. A mechanism for repression of class II gene transcription through specific binding of NC2 to TBP-promoter complexes via heterodimeric histone fold domains. EMBO J. 15: 3105–3116. [PMC free article] [PubMed] [Google Scholar]
- Gumbs, O. H., A. M. Campbell and P. A. Weil, 2003. High-affinity DNA binding by a Mot1p-TBP complex: implications for TAF-independent transcription. EMBO J. 22: 3131–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampsey, M., 1998. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol. Mol. Biol. Rev. 62: 465–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. W., and D. J. Stillman, 1996. Epigenetic effects on yeast transcription caused by mutations in an actin-related protein present in the nucleus. Genes Dev. 10: 604–619. [DOI] [PubMed] [Google Scholar]
- Kobayashi, A., T. Miyake, Y. Ohyama, M. Kawaichi and T. Kokubo, 2001. Mutations in the TATA-binding protein, affecting transcriptional activation, show synthetic lethality with the TAF145 gene lacking the TAF N-terminal domain in Saccharomyces cerevisiae. J. Biol. Chem. 276: 395–405. [DOI] [PubMed] [Google Scholar]
- Kokubo, T., M. J. Swanson, J. I. Nishikawa, A. G. Hinnebusch and Y. Nakatani, 1998. The yeast TAF145 inhibitory domain and TFIIA competitively bind to TATA-binding protein. Mol. Cell. Biol. 18: 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruppa, M., R. D. Moir, D. Kolodrubetz and I. M. Willis, 2001. Nhp6, an HMG1 protein, functions in SNR6 transcription by RNA polymerase III in S. cerevisiae. Mol. Cell 7: 309–318. [DOI] [PubMed] [Google Scholar]
- Lee, T. I., and R. A. Young, 1998. Regulation of gene expression by TBP-associated proteins. Genes Dev. 12: 1398–1408. [DOI] [PubMed] [Google Scholar]
- Lee, T. I., H. C. Causton, F. C. Holstege, W. C. Shen, N. Hannett et al., 2000. Redundant roles for the TFIID and SAGA complexes in global transcription. Nature 405: 701–704. [DOI] [PubMed] [Google Scholar]
- Lemaire, M., and M. A. Collart, 2000. The TATA-binding protein-associated factor yTafII19p functionally interacts with components of the global transcriptional regulator Ccr4-Not complex and physically interacts with the Not5 subunit. J. Biol. Chem. 275: 26925–26934. [DOI] [PubMed] [Google Scholar]
- Lenssen, E., N. James, I. Pedruzzi, F. Dubouloz, E. Cameroni et al., 2005. The Ccr4-Not complex independently controls both Msn2-dependent transcriptional activation—via a newly identified Glc7/Bud14 type I protein phosphatase module—and TFIID promoter distribution. Mol. Cell. Biol. 25: 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. Y., A. Virbasius, X. Zhu and M. R. Green, 1999. Enhancement of TBP binding by activators and general transcription factors. Nature 399: 605–609. [DOI] [PubMed] [Google Scholar]
- Liu, H. Y., V. Badarinarayana, D. C. Audino, J. Rappsilber, M. Mann et al., 1998. The NOT proteins are part of the CCR4 transcriptional complex and affect gene expression both positively and negatively. EMBO J. 17: 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, S., M. Livingstone-Zatchej, S. Jourdain, F. Thoma, A. Sentenac et al., 2001. High-mobility-group proteins NHP6A and NHP6B participate in activation of the RNA polymerase III SNR6 gene. Mol. Cell. Biol. 21: 3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison, J. M., and F. Winston, 1997. Evidence that Spt3 functionally interacts with Mot1, TFIIA, and TATA-binding protein to confer promoter-specific transcriptional control in Saccharomyces cerevisiae. Mol. Cell. Biol. 17: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet, L., C. Tu, Y. K. Hong, E. O. Shuster and M. A. Collart, 2000. The essential function of Not1 lies within the Ccr4-Not complex. J. Mol. Biol. 303: 131–143. [DOI] [PubMed] [Google Scholar]
- Marcus, G. A., N. Silverman, S. L. Berger, J. Horiuchi and L. Guarente, 1994. Functional similarity and physical association between GCN5 and ADA2: putative transcriptional adaptors. EMBO J. 13: 4807–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, M. P., V. L. Gerlach and D. A. Brow, 2001. A novel upstream RNA polymerase III promoter element becomes essential when the chromatin structure of the yeast U6 RNA gene is altered. Mol. Cell. Biol. 21: 6429–6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein, F., K. Yeung, J. Cao, J. A. Inostroza, H. Erdjument-Bromage et al., 1996. Requirement of a corepressor for Dr1-mediated repression of transcription. Genes Dev. 10: 1033–1048. [DOI] [PubMed] [Google Scholar]
- Moreira, J. M., and S. Holmberg, 2000. Chromatin-mediated transcriptional regulation by the yeast architectural factors NHP6A and NHP6B. EMBO J. 19: 6804–6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer, J., L. E. Lezina, J. Ewing, S. Audi and P. M. Lieberman, 1998. Association of transcription factor IIA with TATA binding protein is required for transcriptional activation of a subset of promoters and cell cycle progression in Saccharomyces cerevisiae. Mol. Cell. Biol. 18: 2559–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull, T. T., M. Carey and R. C. Johnson, 1996. Yeast HMG proteins NHP6A/B potentiate promoter-specific transcriptional activation in vivo and assembly of preinitiation complexes in vitro. Genes Dev. 10: 2769–2781. [DOI] [PubMed] [Google Scholar]
- Pereira, L. A., M. P. Klejman and H. T. Timmers, 2003. Roles for BTAF1 and Mot1p in dynamics of TATA-binding protein and regulation of RNA polymerase II transcription. Gene 315: 1–13. [DOI] [PubMed] [Google Scholar]
- Poon, D., A. M. Campbell, Y. Bai and P. A. Weil, 1994. Yeast Taf170 is encoded by MOT1 and exists in a TATA box-binding protein (TBP)-TBP-associated factor complex distinct from transcription factor IID. J. Biol. Chem. 269: 23135–23140. [PubMed] [Google Scholar]
- Prelich, G., and F. Winston, 1993. Mutations that suppress the deletion of an upstream activating sequence in yeast: involvement of a protein kinase and histone H3 in repressing transcription in vivo. Genetics 135: 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh, B. F., 2000. Control of gene expression through regulation of the TATA-binding protein. Gene 255: 1–14. [DOI] [PubMed] [Google Scholar]
- Rothstein, R., 1991. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 194: 281–302. [DOI] [PubMed] [Google Scholar]
- Sanders, S. L., J. Jennings, A. Canutescu, A. J. Link and P. A. Weil, 2002. Proteomics of the eukaryotic transcription machinery: identification of proteins associated with components of yeast TFIID by multidimensional mass spectrometry. Mol. Cell. Biol. 22: 4723–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F., 1991. Getting started with yeast. Methods Enzymol. 194: 1–21. [DOI] [PubMed] [Google Scholar]
- Sikorski, R. S., and P. Hieter, 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stargell, L. A., and K. Struhl, 1995. The TBP-TFIIA interaction in the response to acidic activators in vivo. Science 269: 75–78. [DOI] [PubMed] [Google Scholar]
- Sterner, D. E., and S. L. Berger, 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64: 435–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner, D. E., P. A. Grant, S. M. Roberts, L. J. Duggan, R. Belotserkovskaya et al., 1999. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA- binding protein interaction. Mol. Cell. Biol. 19: 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, M. J., H. Qiu, L. Sumibcay, A. Krueger, S. J. Kim et al., 2003. A multiplicity of coactivators is required by Gcn4p at individual promoters in vivo. Mol. Cell. Biol. 23: 2800–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, B. J., and R. Rothstein, 1989. Elevated recombination rates in transcriptionally active DNA. Cell 56: 619–630. [DOI] [PubMed] [Google Scholar]
- Topalidou, I., M. Papamichos-Chronakis, G. Thireos and D. Tzamarias, 2004. Spt3 and Mot1 cooperate in nucleosome remodeling independently of TBP recruitment. EMBO J. 23: 1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers, A. A., 2003. Priming the nucleosome: A role for HMGB proteins? EMBO Rep. 4: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, M., M. A. Valencia-Sanchez, R. R. Staples, J. Chen, C. L. Denis et al., 2001. The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell 104: 377–386. [DOI] [PubMed] [Google Scholar]
- Tucker, M., R. R. Staples, M. A. Valencia-Sanchez, D. Muhlrad and R. Parker, 2002. Ccr4p is the catalytic subunit of a Ccr4p/Pop2p/Notp mRNA deadenylase complex in Saccharomyces cerevisiae. EMBO J. 21: 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oevelen, C. J., H. A. van Teeffelen and H. T. Timmers, 2005. Differential requirement of SAGA subunits for Mot1p and Taf1p recruitment in gene activation. Mol. Cell. Biol. 25: 4863–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voth, W. P., Y. W. Jiang and D. J. Stillman, 2003. New ‘marker swap’ plasmids for converting selectable markers on budding yeast gene disruptions and plasmids. Yeast 20: 985–993. [DOI] [PubMed] [Google Scholar]
- Winston, F., and P. Sudarsanam, 1998. The SAGA of Spt proteins and transcriptional analysis in yeast: past, present, and future. Cold Spring Harbor Symp. Quant. Biol. 63: 553–561. [DOI] [PubMed] [Google Scholar]
- Yu, Y., P. Eriksson and D. J. Stillman, 2000. Architectural transcription factors and the SAGA complex function in parallel pathways to activate transcription. Mol. Cell. Biol. 20: 2350–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y., P. Eriksson, L. T. Bhoite and D. J. Stillman, 2003. Regulation of TATA-binding protein binding by the SAGA complex and the Nhp6 high-mobility group protein. Mol. Cell. Biol. 23: 1910–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]