Abstract
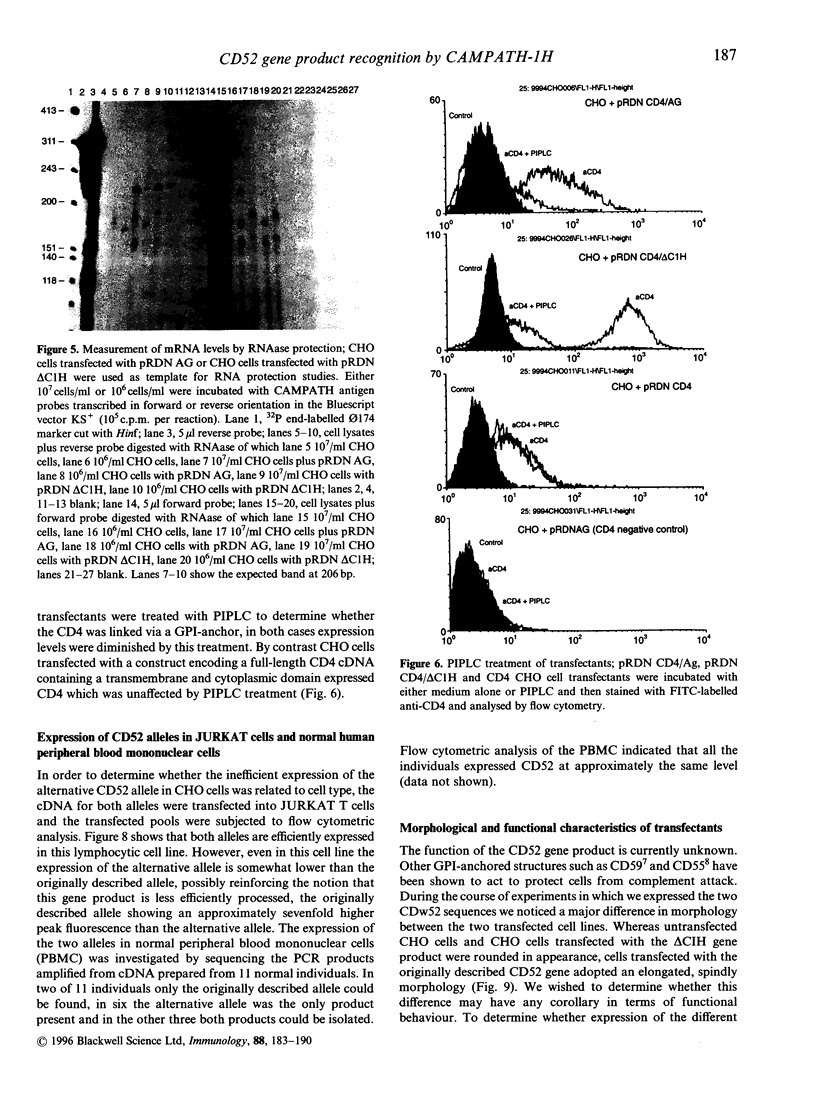
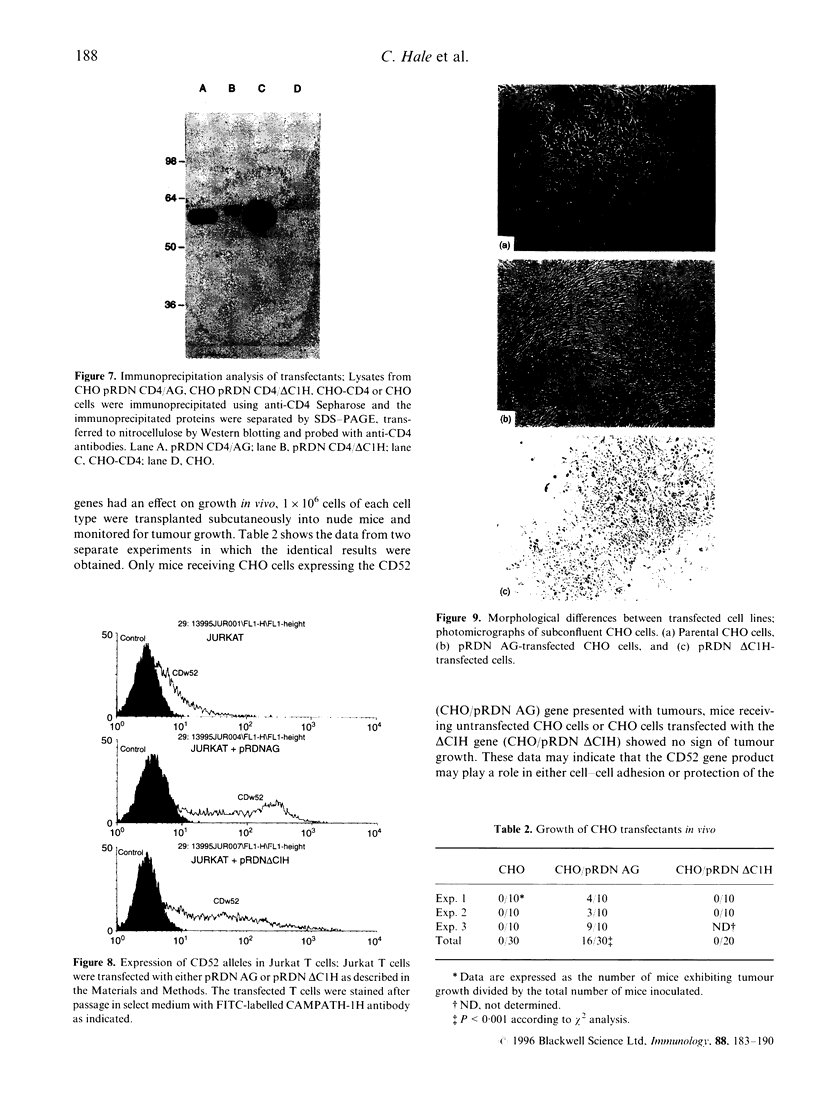
Cloning of the CD52 from a B-lymphocyte tumour cDNA library revealed two closely related sequences differing only at two amino acids C-terminal to the proposed point of glycosylphosphatidylinositol (GPI)-linkage. When transfected into CHO cells only one of these sequences gave high-level expression of the antigen recognized by the prototypic anti-CD52 antibody CAMPATH-1 whereas in JURKAT cells good expression levels were obtained with both sequences. Fusion of the sequence from the second sequence to DNA encoding the extracellular domain of CD4 indicated that this sequence was capable of directing GPI linkage. The possible implications for the function of CD52 and serotherapy with anti-CD52 antibodies are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert M., Kubar J., Bernard A. CD58 and CD59 molecules exhibit potentializing effects in T cell adhesion and activation. J Immunol. 1992 Feb 1;148(3):672–677. [PubMed] [Google Scholar]
- Dyer M. J., Hale G., Hayhoe F. G., Waldmann H. Effects of CAMPATH-1 antibodies in vivo in patients with lymphoid malignancies: influence of antibody isotype. Blood. 1989 May 1;73(6):1431–1439. [PubMed] [Google Scholar]
- Hale G., Xia M. Q., Tighe H. P., Dyer M. J., Waldmann H. The CAMPATH-1 antigen (CDw52). Tissue Antigens. 1990 Mar;35(3):118–127. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- Isaacs J. D., Watts R. A., Hazleman B. L., Hale G., Keogan M. T., Cobbold S. P., Waldmann H. Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet. 1992 Sep 26;340(8822):748–752. doi: 10.1016/0140-6736(92)92294-p. [DOI] [PubMed] [Google Scholar]
- Kirchhoff C., Krull N., Pera I., Ivell R. A major mRNA of the human epididymal principal cells, HE5, encodes the leucocyte differentiation CDw52 antigen peptide backbone. Mol Reprod Dev. 1993 Jan;34(1):8–15. doi: 10.1002/mrd.1080340103. [DOI] [PubMed] [Google Scholar]
- Kodukula K., Gerber L. D., Amthauer R., Brink L., Udenfriend S. Biosynthesis of glycosylphosphatidylinositol (GPI)-anchored membrane proteins in intact cells: specific amino acid requirements adjacent to the site of cleavage and GPI attachment. J Cell Biol. 1993 Feb;120(3):657–664. doi: 10.1083/jcb.120.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Caras I. W. Fusion of sequence elements from non-anchored proteins to generate a fully functional signal for glycophosphatidylinositol membrane anchor attachment. J Cell Biol. 1991 Dec;115(6):1595–1600. doi: 10.1083/jcb.115.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Kwong J., Fujita T., Olszowy M. W., Shaw A. S., Lublin D. M. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J Immunol. 1992 Dec 1;149(11):3535–3541. [PubMed] [Google Scholar]
- Treumann A., Lifely M. R., Schneider P., Ferguson M. A. Primary structure of CD52. J Biol Chem. 1995 Mar 17;270(11):6088–6099. doi: 10.1074/jbc.270.11.6088. [DOI] [PubMed] [Google Scholar]
- Xia M. Q., Hale G., Lifely M. R., Ferguson M. A., Campbell D., Packman L., Waldmann H. Structure of the CAMPATH-1 antigen, a glycosylphosphatidylinositol-anchored glycoprotein which is an exceptionally good target for complement lysis. Biochem J. 1993 Aug 1;293(Pt 3):633–640. doi: 10.1042/bj2930633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M. Q., Tone M., Packman L., Hale G., Waldmann H. Characterization of the CAMPATH-1 (CDw52) antigen: biochemical analysis and cDNA cloning reveal an unusually small peptide backbone. Eur J Immunol. 1991 Jul;21(7):1677–1684. doi: 10.1002/eji.1830210714. [DOI] [PubMed] [Google Scholar]