Abstract
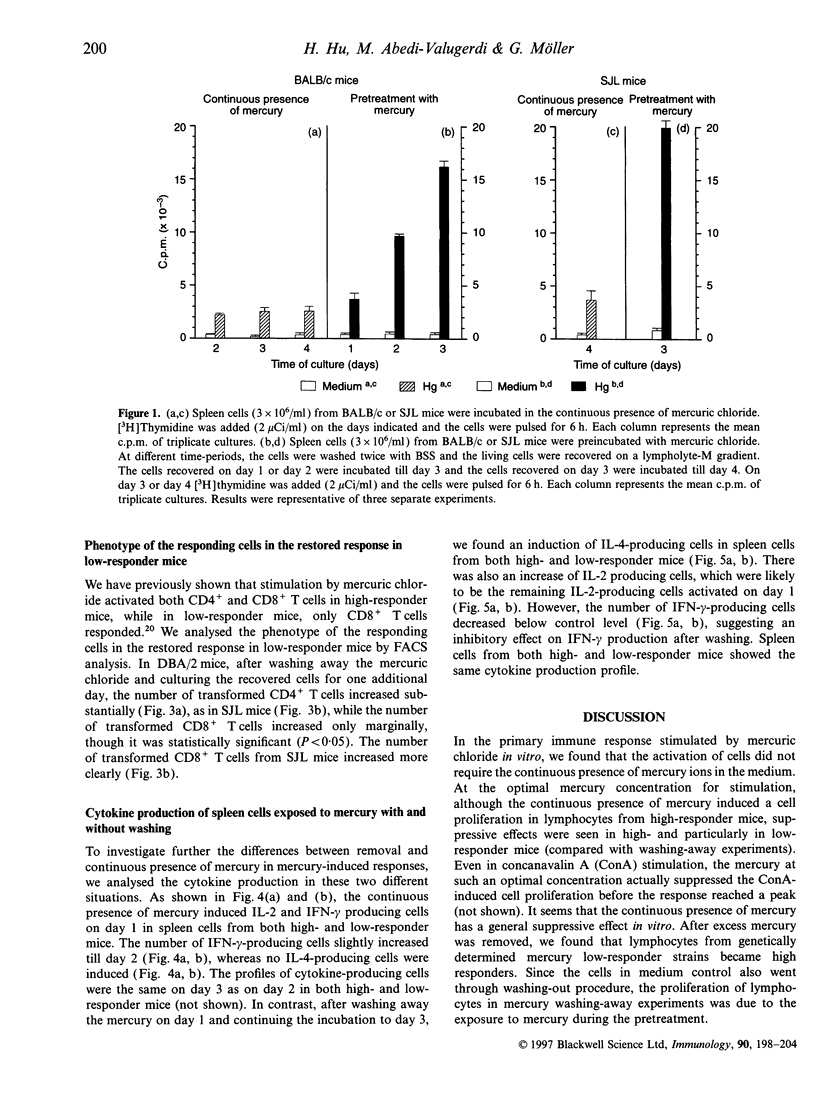
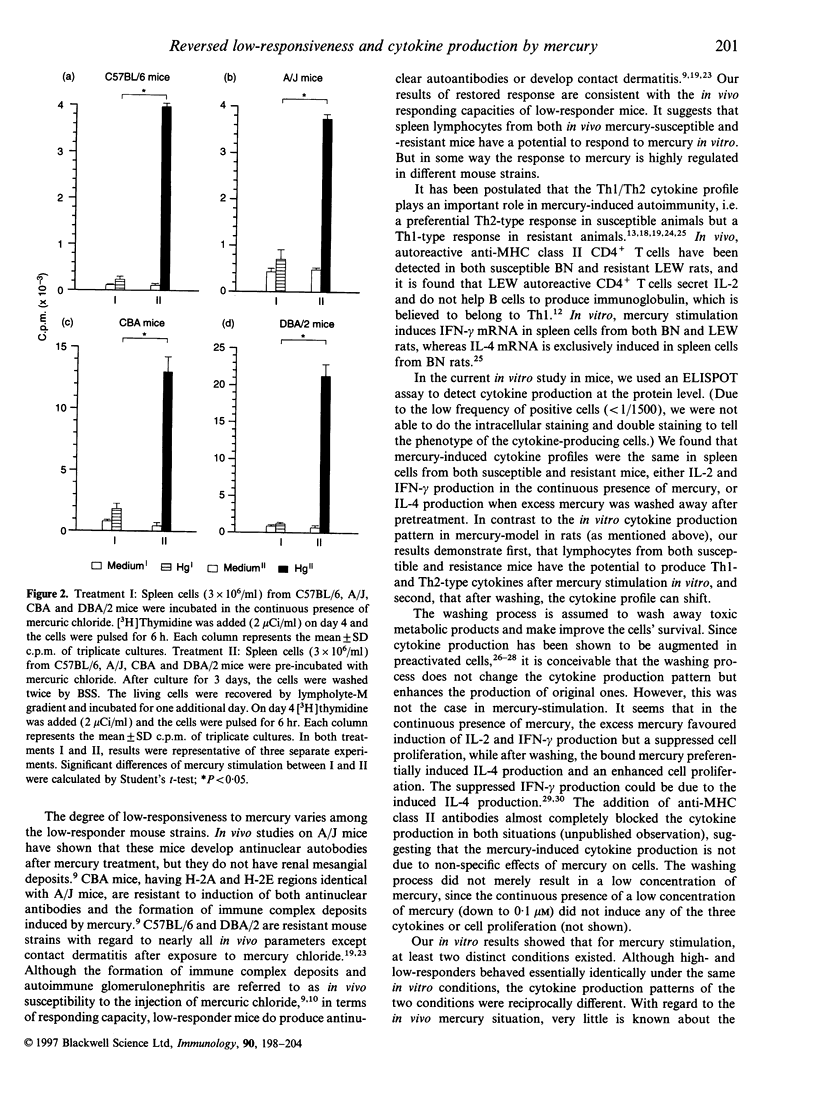
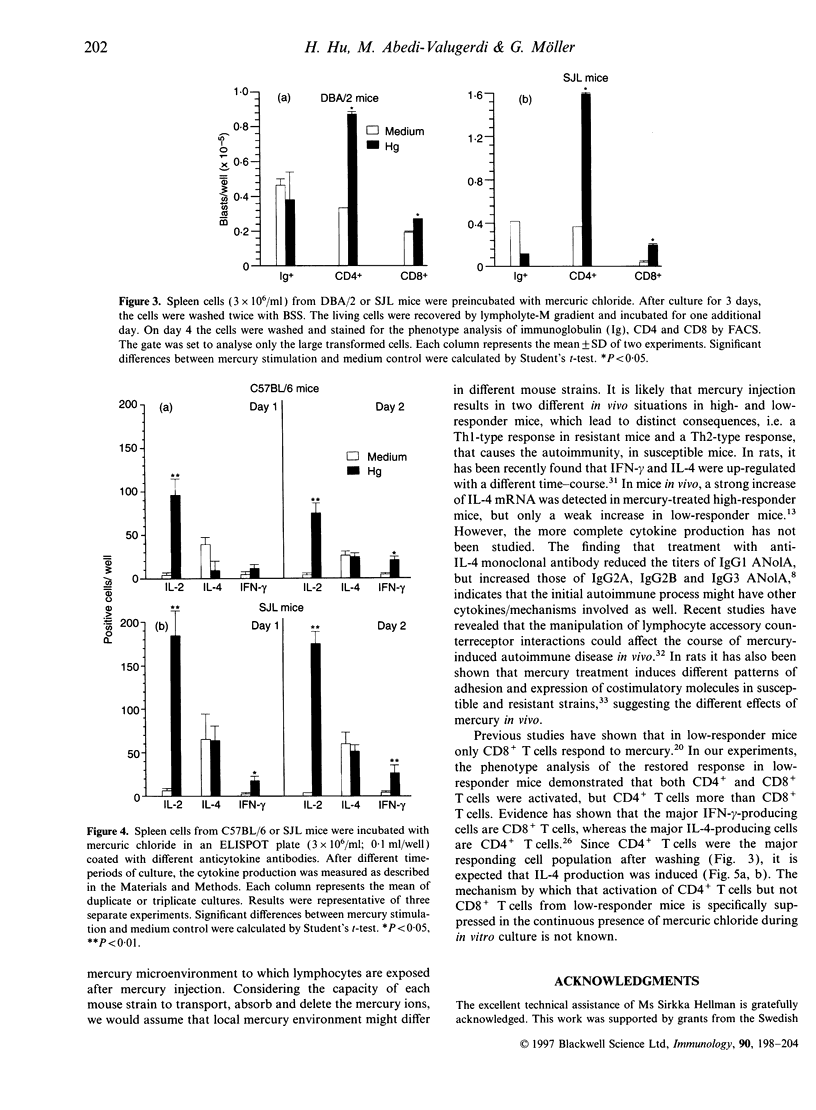
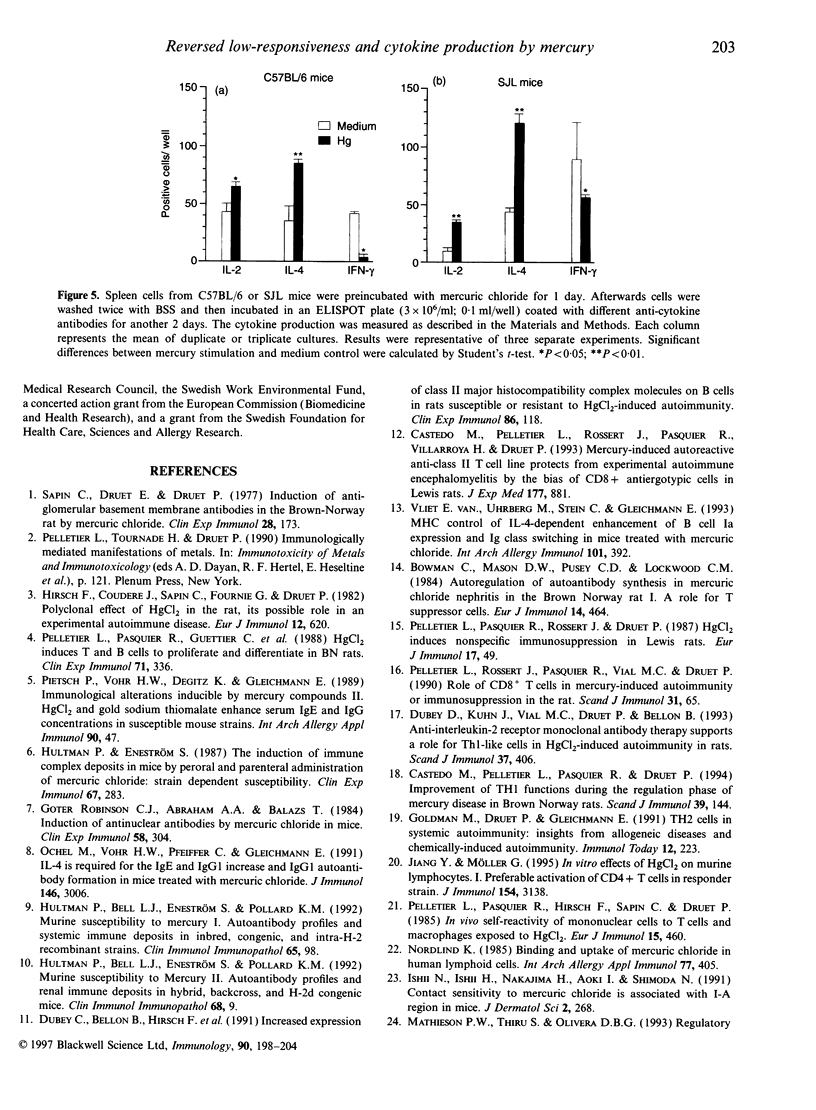
Mercury can induce autoimmune disease in susceptible mouse strains. We found that in vitro mercuric chloride induced a high proliferative response in spleen lymphocytes from mercury-susceptible SJL mice, but a low response in resistant mice, such as C57BL/6 (H-2b), A/J (H-2a) and CBA (H-2k) mice. However, a high proliferative response was obtained with lymphocytes from all tested low-responder mice by pretreating them in vitro for 1-3 days with mercuric chloride and then wash away the excess mercury. Both CD4+ and CD8+ T cells were activated in the restored response, but CD4+ T cells was the major responding cell population, as in high-responder mice. We also measured the cytokine production at the protein level after mercury stimulation in vitro. We found that in mercury stimulation the different culture conditions resulted in different patterns of cytokine production. The continuous presence of mercury induced interleukin-2 (IL-2) and interferon-gamma, but not IL-4 production in spleen cells from both high- and low-responder mice. In contrast, by pretreating the cells with mercury and then washing, spleen cells from both high and low-responder mice produced IL-4. Our results suggest that spleen cells from both mercury-susceptible and -resistant mice have the potential to respond to mercury in vitro and produce both Th1- and Th2-type cytokines. But the mercury-induced cytokine profile can shift depending on the conditions for activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biancone L., Andres G., Ahn H., Lim A., Dai C., Noelle R., Yagita H., De Martino C., Stamenkovic I. Distinct regulatory roles of lymphocyte costimulatory pathways on T helper type-2 mediated autoimmune disease. J Exp Med. 1996 Apr 1;183(4):1473–1481. doi: 10.1084/jem.183.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C., Mason D. W., Pusey C. D., Lockwood C. M. Autoregulation of autoantibody synthesis in mercuric chloride nephritis in the Brown Norway rat. I. A role for T suppressor cells. Eur J Immunol. 1984 May;14(5):464–470. doi: 10.1002/eji.1830140515. [DOI] [PubMed] [Google Scholar]
- Castedo M., Pelletier L., Pasquier R., Druet P. Improvement of TH1 functions during the regulation phase of mercury disease in brown Norway rats. Scand J Immunol. 1994 Feb;39(2):144–150. doi: 10.1111/j.1365-3083.1994.tb03353.x. [DOI] [PubMed] [Google Scholar]
- Castedo M., Pelletier L., Rossert J., Pasquier R., Villarroya H., Druet P. Mercury-induced autoreactive anti-class II T cell line protects from experimental autoimmune encephalomyelitis by the bias of CD8+ antiergotypic cells in Lewis rats. J Exp Med. 1993 Apr 1;177(4):881–889. doi: 10.1084/jem.177.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey C., Bellon B., Hirsch F., Kuhn J., Vial M. C., Goldman M., Druet P. Increased expression of class II major histocompatibility complex molecules on B cells in rats susceptible or resistant to HgCl2-induced autoimmunity. Clin Exp Immunol. 1991 Oct;86(1):118–123. doi: 10.1111/j.1365-2249.1991.tb05783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey D., Kuhn J., Vial M. C., Druet P., Bellon B. Anti-interleukin-2 receptor monoclonal antibody therapy supports a role for Th1-like cells in HgCl2-induced autoimmunity in rats. Scand J Immunol. 1993 Apr;37(4):406–412. doi: 10.1111/j.1365-3083.1993.tb03311.x. [DOI] [PubMed] [Google Scholar]
- Goldman M., Druet P., Gleichmann E. TH2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991 Jul;12(7):223–227. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Hultman P., Bell L. J., Eneström S., Pollard K. M. Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic, and intra-H-2 recombinant strains. Clin Immunol Immunopathol. 1992 Nov;65(2):98–109. doi: 10.1016/0090-1229(92)90212-7. [DOI] [PubMed] [Google Scholar]
- Hultman P., Bell L. J., Eneström S., Pollard K. M. Murine susceptibility to mercury. II. autoantibody profiles and renal immune deposits in hybrid, backcross, and H-2d congenic mice. Clin Immunol Immunopathol. 1993 Jul;68(1):9–20. doi: 10.1006/clin.1993.1088. [DOI] [PubMed] [Google Scholar]
- Hultman P., Eneström S. The induction of immune complex deposits in mice by peroral and parenteral administration of mercuric chloride: strain dependent susceptibility. Clin Exp Immunol. 1987 Feb;67(2):283–292. [PMC free article] [PubMed] [Google Scholar]
- Höidén I., Möller G. Interferon-gamma and growth factor production by murine T cells derived from three different lymphoid tissues. Eur J Immunol. 1991 Nov;21(11):2703–2709. doi: 10.1002/eji.1830211109. [DOI] [PubMed] [Google Scholar]
- Ishii N., Ishii H., Nakajima H., Aoki I., Shimoda N. Contact sensitivity to mercuric chloride is associated with I-A region in mice. J Dermatol Sci. 1991 Jul;2(4):268–273. doi: 10.1016/0923-1811(91)90050-8. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Möller G. In vitro effects of HgCl2 on murine lymphocytes. I. Preferable activation of CD4+ T cells in a responder strain. J Immunol. 1995 Apr 1;154(7):3138–3146. [PubMed] [Google Scholar]
- Mathieson P. W. Mercury: god of Th2 cells? Clin Exp Immunol. 1995 Nov;102(2):229–230. doi: 10.1111/j.1365-2249.1995.tb03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlind K. Binding and uptake of mercuric chloride in human lymphoid cells. Int Arch Allergy Appl Immunol. 1985;77(4):405–408. doi: 10.1159/000233816. [DOI] [PubMed] [Google Scholar]
- Ochel M., Vohr H. W., Pfeiffer C., Gleichmann E. IL-4 is required for the IgE and IgG1 increase and IgG1 autoantibody formation in mice treated with mercuric chloride. J Immunol. 1991 May 1;146(9):3006–3011. [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Guettier C., Vial M. C., Mandet C., Nochy D., Bazin H., Druet P. HgC12 induces T and B cells to proliferate and differentiate in BN rats. Clin Exp Immunol. 1988 Feb;71(2):336–342. [PMC free article] [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Hirsch F., Sapin C., Druet P. In vivo self-reactivity of mononuclear cells to T cells and macrophages exposed to HgCl2. Eur J Immunol. 1985 May;15(5):460–465. doi: 10.1002/eji.1830150509. [DOI] [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Rossert J., Druet P. HgCl2 induces nonspecific immunosuppression in Lewis rats. Eur J Immunol. 1987 Jan;17(1):49–54. doi: 10.1002/eji.1830170109. [DOI] [PubMed] [Google Scholar]
- Pelletier L., Rossert J., Pasquier R., Vial M. C., Druet P. Role of CD8+ T cells in mercury-induced autoimmunity or immunosuppression in the rat. Scand J Immunol. 1990 Jan;31(1):65–74. doi: 10.1111/j.1365-3083.1990.tb02744.x. [DOI] [PubMed] [Google Scholar]
- Prigent P., Saoudi A., Pannetier C., Graber P., Bonnefoy J. Y., Druet P., Hirsch F. Mercuric chloride, a chemical responsible for T helper cell (Th)2-mediated autoimmunity in brown Norway rats, directly triggers T cells to produce interleukin-4. J Clin Invest. 1995 Sep;96(3):1484–1489. doi: 10.1172/JCI118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Claessen N., Weening J. J., Aten J. Enhanced T lymphocyte expression of LFA-1, ICAM-1, and the TNF receptor family member OX40 in HgCl2-induced systemic autoimmunity. Scand J Immunol. 1996 May;43(5):507–518. doi: 10.1046/j.1365-3083.1996.d01-66.x. [DOI] [PubMed] [Google Scholar]
- Sander B., Cardell S., Möller E. Interleukin 4 and interferon gamma production in restimulated CD4+ and CD8+ cells indicates memory type responsiveness. Scand J Immunol. 1991 Mar;33(3):287–296. doi: 10.1111/j.1365-3083.1991.tb01774.x. [DOI] [PubMed] [Google Scholar]
- Sapin C., Druet E., Druet P. Induction of anti-glomerular basement membrane antibodies in the Brown-Norway rat by mercuric chloride. Clin Exp Immunol. 1977 Apr;28(1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., McKenzie D. T., Weinberg A. D., Hancock W. Characterization of T helper 1 and 2 cell subsets in normal mice. Helper T cells responsible for IL-4 and IL-5 production are present as precursors that require priming before they develop into lymphokine-secreting cells. J Immunol. 1988 Nov 15;141(10):3445–3455. [PubMed] [Google Scholar]
- Swain S. L., Weinberg A. D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990 Dec 1;145(11):3796–3806. [PubMed] [Google Scholar]
- van Vliet E., Uhrberg M., Stein C., Gleichmann E. MHC control of IL-4-dependent enhancement of B cell Ia expression and Ig class switching in mice treated with mercuric chloride. Int Arch Allergy Immunol. 1993;101(4):392–401. doi: 10.1159/000236482. [DOI] [PubMed] [Google Scholar]


