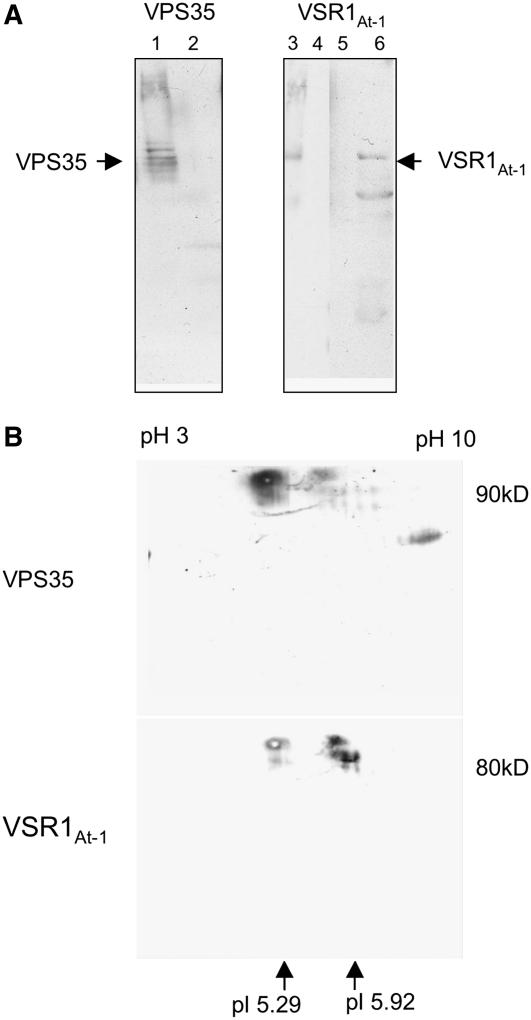
Figure 11.
VSRAt-1 Coimmunoprecipitates with Vps35 Antibodies.
(A) Fractions 5 and 6 (47 to 50% [w/w] sucrose) from an Arabidopsis P100 linear sucrose gradient were treated with CHAPS and subsequently centrifuged at 100,000g for 1 h. The supernatant was then subjected to immunoprecipitation with either VPS35 or VSRAt-1 antibodies covalently coupled to Sepharose CL-6B beads. Bound proteins were dissociated from the beads with glycine, pH 2.5, neutralized, and precipitated. Lane 1, positive control showing the precipitation of VPS35 with VPS35 antibodies; lane 2, negative control using beads coated with a nonspecific antibody (anti-γ-COP); lane 3, marker lane with detergent-solubilized membranes; lane 4, negative control as for lane 2; lane 5, negative control with uncoated beads; lane 6, eluted proteins bound to VPS35-coated beads. Protein gel blots were probed with VPS35 (lane 1) or VSRAt-1 antibodies (lanes 2 and 3). Lanes were loaded with equal amounts of protein (20 μg).
(B) VPS35 immunoprecipitated proteins from detergent-solubilized high-density P100 membranes (as in [A]) were subjected to 2D gel electrophoresis (first isoelectric focusing in a stabilized linear pH gradient followed by size separation in a 10% polyacrylamide gel) before protein gel blotting. In the 2D blot probed with VPS35 antibodies, a signal (arrow) was detected at the expected size (∼90 kD) and at the expected pI of 5.29. The blot was reprobed, without stripping, with VSRAt-1 antisera, and a signal (arrow) was seen at the expected size (∼80 kD) and at the expected pI of 5.92. Since both antisera were made in rabbits, a residual VPS35 signal was still present in (B).