Figure 3.
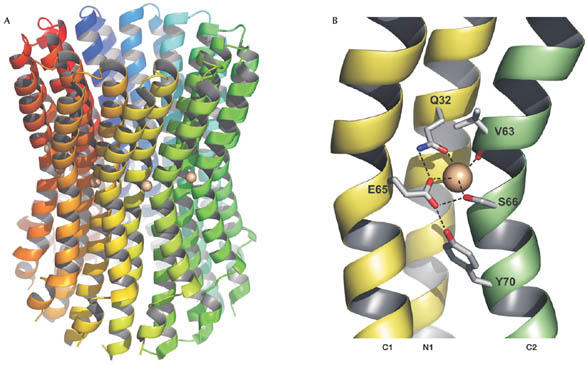
Structure of the I. tartaricus c11 ring in ribbon form. (A) Individual subunits are shown in different colors. The grey spheres indicate the bound Na+ ions. The structure shows a cylindrical, hourglass-shaped protein complex with an outer diameter of ∼45 Å in the middle of the membrane and ∼50 Å at the top and bottom. The ring has a height of ∼70 Å and therefore protrudes out of the membrane on both sides. A hydrophobic cavity with a diameter of ∼17 Å at its narrowest part is located in the centre of the ring. (B) Close-up of the Na+ binding site formed by the inner (N1) and outer helix (C1) of one c-subunit and the outer helix (C2) of the neighbouring c-subunit. Na+ coordination and selected hydrogen bonds are indicated with dashed lines. This structure shows the locked conformation. During opening, the side chain of Y70 might relocate into a cavity underneath the binding site, thus destabilizing the hydrogen bonding network and allowing unloading and loading of the binding site to and from subunit a.
