Figure 1.
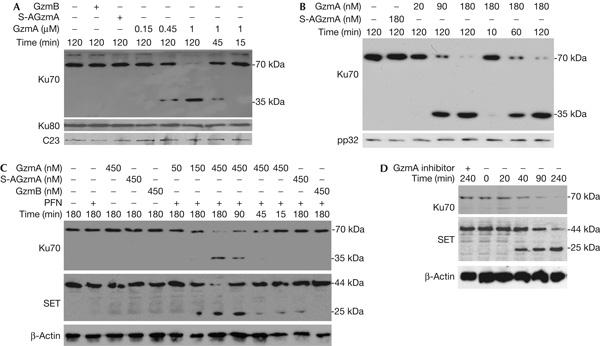
Ku70 is a granzyme A substrate. (A) Granzyme A (GzmA) cleaves Ku70, but not Ku80, in isolated nuclei. HeLa nuclei were incubated with indicated concentrations of GzmA or 1 μM S-AGzmA or GzmB at 37°C for indicated durations and analysed by immunoblot for Ku70, Ku80 or C23, a loading control. GzmA cuts Ku70 to generate a 35 kDa carboxy-terminal fragment, but Ku80 is not cleaved. Neither enzymatically inactive S-AGzmA nor GzmB cuts Ku70 or Ku80. (B) GzmA cuts recombinant Ku70. GzmA or inactive S-AGzmA was added to 0.5 μM Ku70 at 37°C for indicated durations and analysed by Ku70 immunoblot. The 35 kDa cleavage product is seen again. (C) GzmA and perforin (PFN) treatment of K562 degrades Ku70 and SET with similar kinetics. GzmA, but not GzmB or S-AGzmA, degrades Ku70 in cells. The 35 kDa cleavage fragment is also shown. (D) Ku70, like SET, is degraded in 40 min of cytotoxic T lymphocyte (CTL) attack. Ku70 and SET were not degraded when CTLs were pretreated with the GzmA inhibitor diisocoumarin. β-Actin is a loading control.
