Figure 3.
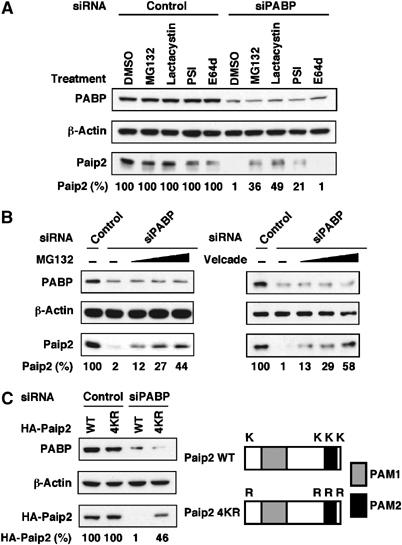
Paip2 levels are regulated by the ubiquitin-proteasome system. (A) Proteasome inhibition causes Paip2 accumulation in PABP-depleted cells. HeLa cells were transfected with control or PABP siRNAs. At 24 h after transfection, cells were treated with either DMSO or with 20 μM each of MG132, Lactacystin, PSI or E64d for 6 h. Cell extracts were then subjected to Western blot analysis with anti-PABP and anti-Paip2 antibodies. Data are expressed as the percentage of Paip2 amount relative to that in control siRNA-transfected cells (set at 100%). (B) At 24 h after siRNA transfection, cells were treated with either DMSO or with increasing concentrations (10, 20 and 40 μM) of MG132 (left panel) or (50, 100 and 200 nM) of Velcade (right panel) for 6 h and then analyzed as in (A). (C) Degradation of Paip2 in PABP knockdown cells is diminished by lysine mutations. Schematic representation of Paip2 WT and Paip2 4KR in which all the lysine residues were replaced by arginines (K2R, K108R, K116R and K123R). Gray and black boxes represent PAM1 and PAM2, respectively. HeLa cells were transfected with the indicated Paip2 plasmids. At 24 h after transfection, cells were transfected with siRNAs and then analyzed as in (A). The results are representative of two independent experiments.
