Figure 6.
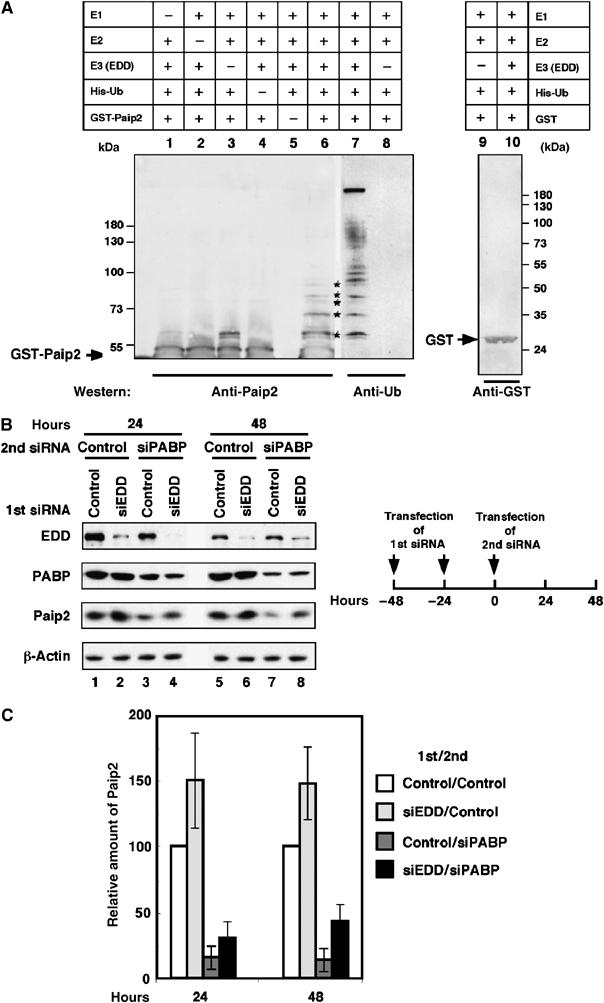
Ubiquitination of Paip2 is mediated by EDD. (A) Ubiquitination of Paip2 by EDD in vitro. EDD, isolated by immunoprecipitation with anti-EDD antibodies from rat testis lysate, was incubated with E1, E2 (UBC4-1), His-ubiquitin, ubiquitin aldehyde, AMP-PNP and either GST-Paip2 or GST. Products were then isolated by using glutathione coupled beads. Higher molecular weight ubiquitinated forms of GST-Paip2 (lane 6, asterisks) were detected by immunoblotting with anti-Paip2. As negative controls, immunoprecipitates obtained with preimmune rabbit IgG were used in the in vitro ubiquitination assay (lane 3) or reactions were carried out with GST as substrate and detected with anti-GST antibodies (lanes 9 and 10). To confirm that the higher molecular weight bands in lane 6 contained ubiquitin, replicates of samples used in lanes 3 and 6 were analyzed by immunoblotting with anti-ubiquitin antibody (lanes 7 and 8). Bands of ubiquitinated GST-Paip2 are marked with asterisks. The positions of GST and GST-Paip2 are indicated by arrows. (B) Depletion of EDD by RNAi prevents Paip2 degradation. HeLa cells were transfected consecutively at a 24-h interval with control or EDD siRNA. At 24 h following the second transfection, cells were transfected with control siRNA or PABP siRNA. At 24 and 48 h after the second siRNA transfection, cell extracts were subjected to Western blot analysis with anti-EDD, anti-PABP and anti-Paip2 antibodies. (C) Histogram of the relative amount of Paip2 from (B). Data are expressed as the percentage of Paip2 amount relative to that in control siRNA-transfected cells (set at 100%). Error bars denote the standard error of four independent experiments.
