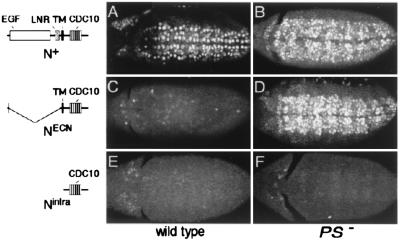
Figure 1.
Presenilin-dependent activity of Notch in the embryonic central nervous system. Neuroblasts marked by the expression of Hb protein are shown in wild-type (A, C, and E) and PS− (B, D, and F) embryos that ubiquitously express either N+ (A and B), NECN (C and D), or Nintra (E and F) under Gal4/UAS control. (A) Ubiquitous expression of N+ in otherwise wild-type embryos has little or no effect on neuroblast segregation. (C and E) In contrast, ubiquitous expression of either NECN or Nintra suppresses neuroblast segregations, indicating constitutive transducing activity. In PS− embryos, most or all ventral ectodermal cells segregate as neuroblasts, even when N+ or NECN are ubiquitously expressed (B and D), indicating that the transducing activity of these transmembrane forms requires Presenilin. In contrast, neuroblast segregation is suppressed in PS− embryos expressing Nintra (F), indicating that this form of Notch bypasses the requirement for Presenilin. All embryos are shown in ventral aspect just after completion of germ-band elongation (anterior to the left). The structure of the Notch proteins are shown schematically: EGF, epidermal growth factor motifs; LNR, LIN-12/Notch repeat motifs; TM, transmembrane domain; CDC10, CDC10/SWI6 motifs.