Abstract
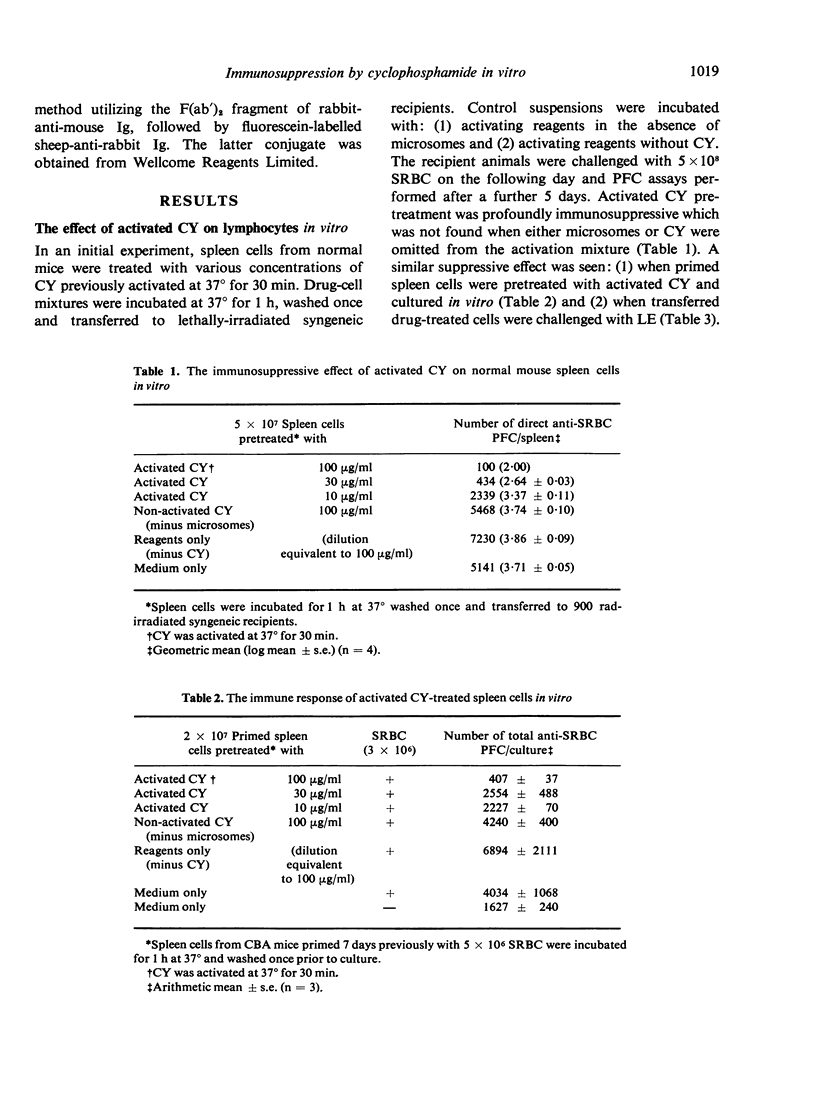
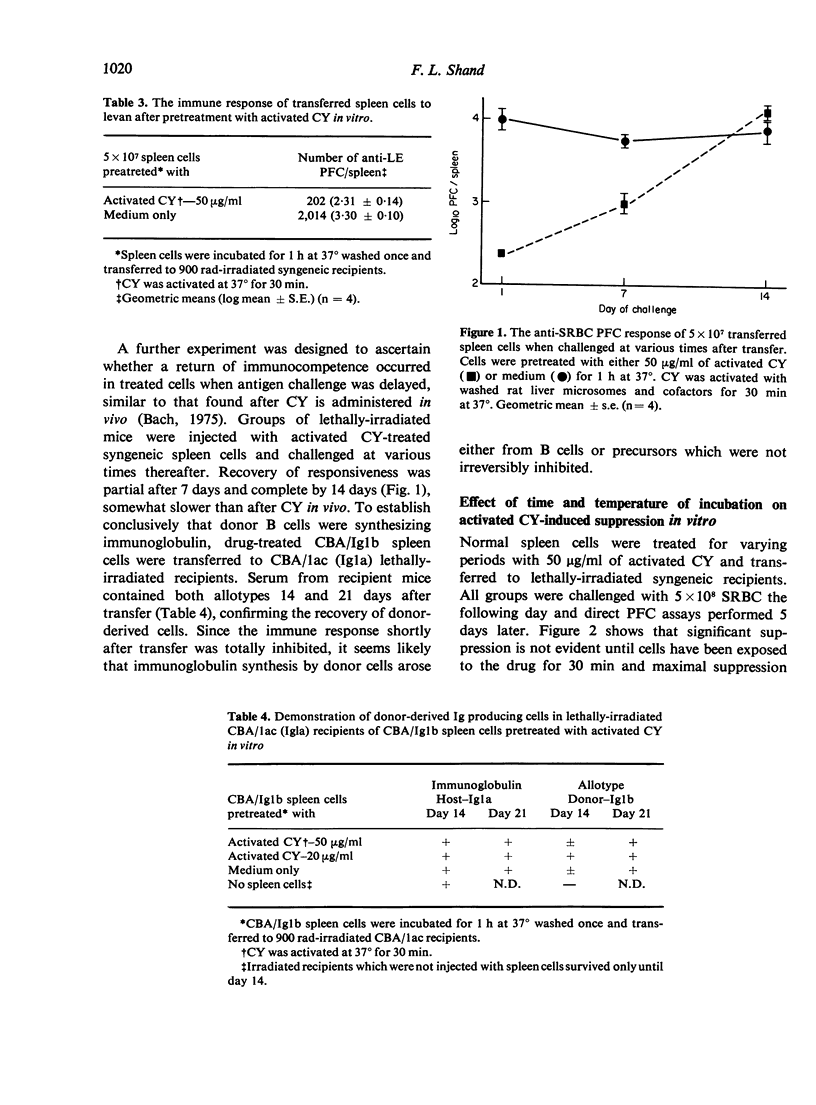
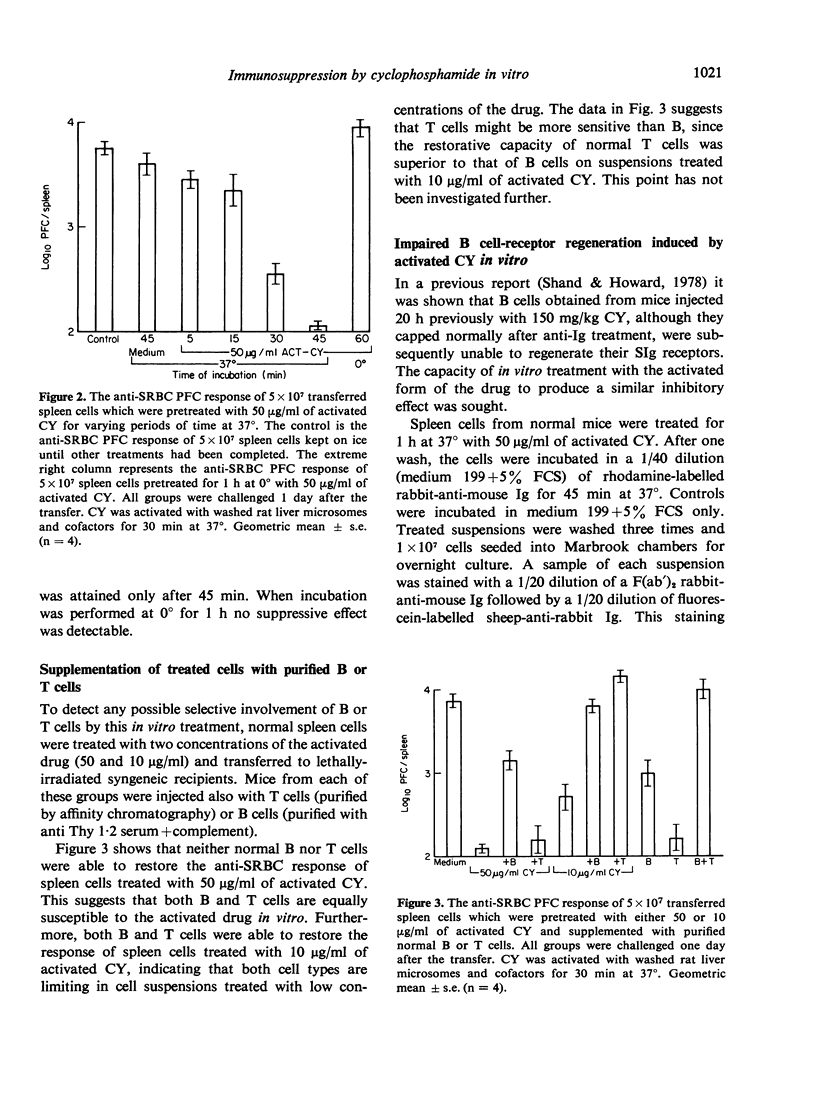
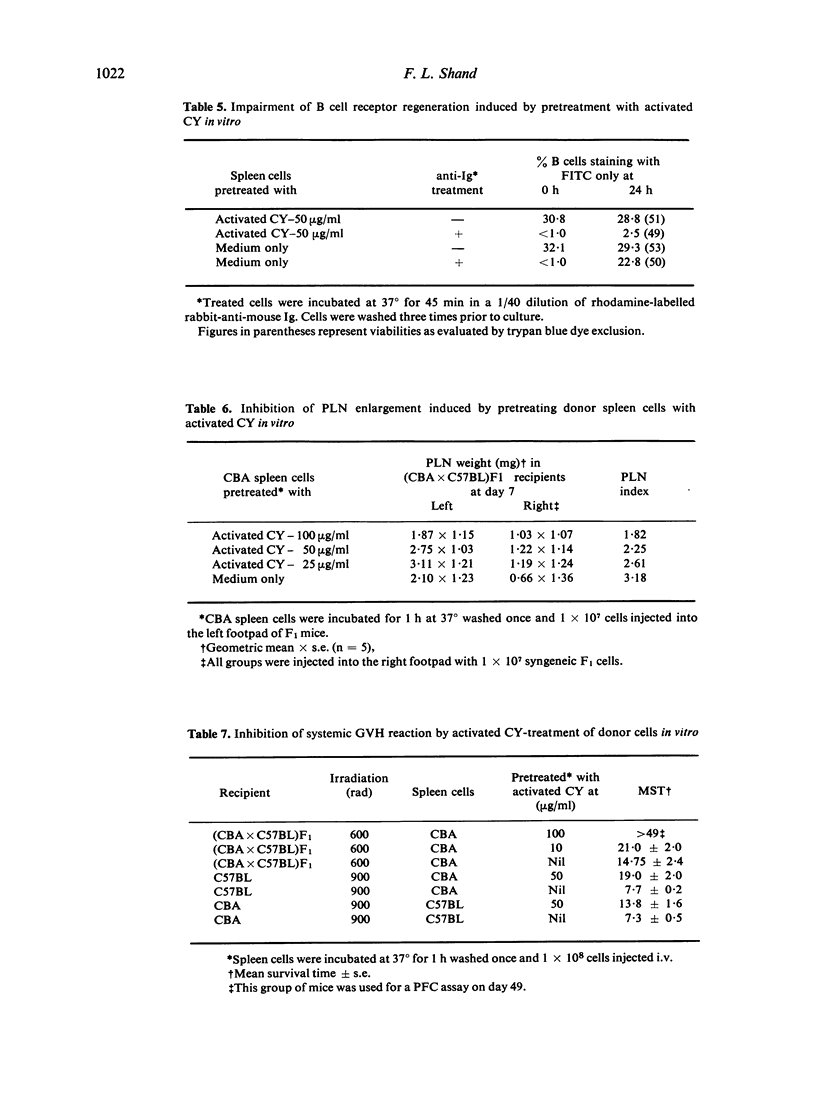
Cyclophosphamide (CY) was activated in vitro with washed rat liver microsomes and cofactors. Pretreatment of mouse spleen cells in vitro with the activated drug abolished their capacity to give a primary antibody response to SRBC and levan on transfer to irradiated syngeneic recipients. However, responsiveness returned if challenge was delayed for 7 or more days after transfer. Part of this was shown to be of donor origin by an allotype marker. The treatment of normal spleen cells with activated CY in vitro also prevented B cells from regenerating their immunoglobulin receptors after capping with anti-immunoglobulin serum. The induction of suppression required contact between lymphocytes and activated CY for at least 30 min at 37 degrees and did not appear following incubation for 1 h at 0 degrees. Since the antibody response of drug-treated spleen cells to SRBC could not be restored with purified normal B or T cells, it is probable that B and T lymphocytes are both susceptible to suppression by activated CY in vitro. Similar pretreatment abrogated the graft-versus-host (GVH) reactivity of spleen cells as measured by survival and in a popliteal lymph node assay. B cell chimerism in F1 recipients of drug-treated parental spleen cells was demonstrated by the presence of congenic allotype markers. This suggests a possible approach for the attenuation of GVH disease which is associated with bone marrow transplantation in man.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK N., HOHORST H. J. UBER DIE AKTIVIERUNG VON CYCLOPHOSPHAMID IN VIVO UND IN VITRO. Arzneimittelforschung. 1963 Dec;13:1021–1031. [PubMed] [Google Scholar]
- Brock N. Comparative pharmacologic study in vitro and in vivo with cyclophosphamide (NSC-26271), cyclophosphamide metabolites, and plain nitrogen mustard compounds. Cancer Treat Rep. 1976 Apr;60(4):301–308. [PubMed] [Google Scholar]
- Connors T. A., Cox P. J., Farmer P. B., Foster A. B., Jarman M. Some studies of the active intermediates formed in the microsomal metabolism of cyclophosphamide and isophosphamide. Biochem Pharmacol. 1974 Jan 1;23(1):115–129. doi: 10.1016/0006-2952(74)90318-9. [DOI] [PubMed] [Google Scholar]
- Connors T. A., Grover P. L., McLoughlin A. M. Microsomal activation of cyclophosphamide in vivo. Biochem Pharmacol. 1970 Apr;19(4):1533–1535. doi: 10.1016/0006-2952(70)90077-8. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Burr W., Simonsen M. A lymph node weight assay for the graft-versus-host activity of rat lymphoid cells. Transplantation. 1970 Sep;10(3):258–266. doi: 10.1097/00007890-197009000-00007. [DOI] [PubMed] [Google Scholar]
- Grover P. L., Sims P. Enzyme-catalysed reactions of polycyclic hydrocarbons with deoxyribonucleic acid and protein in vitro. Biochem J. 1968 Nov;110(1):159–160. doi: 10.1042/bj1100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman S. P., Weidanz W. P. The effect of cyclophosphamide on the ontogeny of the humoral immune response in chickens. J Immunol. 1970 Sep;105(3):614–619. [PubMed] [Google Scholar]
- Many A., Schwartz R. S. Drug-induced immunologic tolerance: site of action of cyclophosphamide. Proc Soc Exp Biol Med. 1970 Mar;133(3):754–757. doi: 10.3181/00379727-133-34558. [DOI] [PubMed] [Google Scholar]
- Many A., Schwartz R. S. Periodicity during recovery of immune response after cyclophosphamide treatment. Blood. 1971 Jun;37(6):692–695. [PubMed] [Google Scholar]
- Miranda J. J. Studies on immunological paralysis. IX. The immunogenicity and tolerogenicity of levan (polyfructose) in mice. Immunology. 1972 Dec;23(6):829–842. [PMC free article] [PubMed] [Google Scholar]
- Owens A. H., Jr, Santos G. W. The effect of cytotoxic drugs on graft-versus-host disease in mice. Transplantation. 1971 Apr;11(4):378–382. doi: 10.1097/00007890-197104000-00004. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Bretscher P. A., Parish C. R. Regulation of the immune response. II. Repressor T cells in cyclophosphamide-induced tolerant mice. Eur J Immunol. 1977 Mar;7(3):180–185. doi: 10.1002/eji.1830070313. [DOI] [PubMed] [Google Scholar]
- Shand F. L. Analysis of immunosuppression generated by the graft-versus-host reaction. I. A suppressor T-cell component studied in vivo. Immunology. 1975 Dec;29(6):953–965. [PMC free article] [PubMed] [Google Scholar]
- Shand F. L. Analysis of immunosuppression generated by the graft-versus-host reaction. II. Characterization of the suppression cell and its mechanism of action. Immunology. 1976 Dec;31(6):943–951. [PMC free article] [PubMed] [Google Scholar]
- Shand F. L., Howard J. G. Cyclophosphamide inhibited B cell receptor regeneration as a basis for drug-induced tolerance;. Nature. 1978 Jan 19;271(5642):255–257. doi: 10.1038/271255a0. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]


