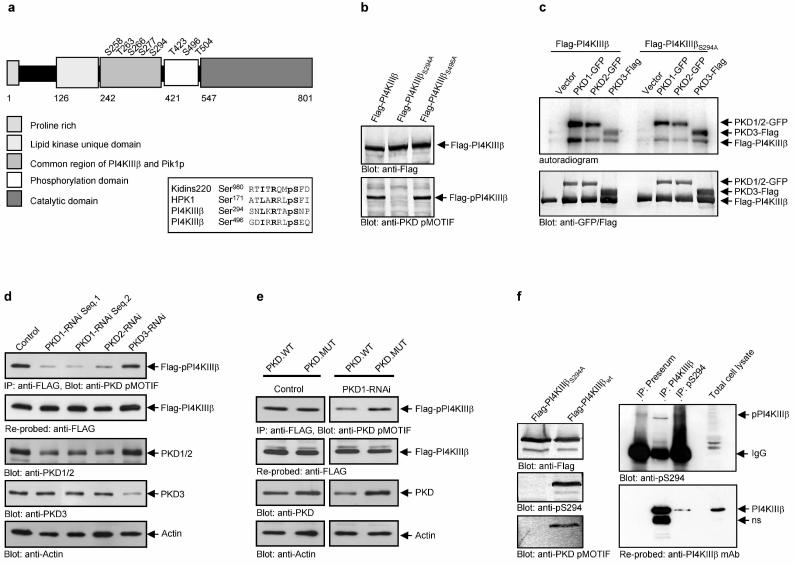
Figure 3.
PKD phosphorylates serine 294 in PI4KIIIβ. (a) Schematic view of the domain structure of PI4KIIIβ and the eight known phosphorylation sites and alignment of the putative PKD phosphorylation sites in PI4KIIIβ, serine 294 and 496 with the PKD substrate Kidins220 (bold: critical amino acids: pS: phospho-serine; R: arginine at −3; L/I: critical leucine or isoleucine at −5) (b) the anti-PKD pMOTIF antibody detects the phosphorylated serine 294. HEK293 cells expressing the indicated proteins were lysed and Flag-PI4KIIIβ was detected by Western Blot using anti-Flag and anti-PKD pMOTIF antibodies. (c) PKD phosphorylates serine 294 in Flag-PI4KIIIβ in vitro. HEK293 cells were transfected with plasmids encoding the indicated proteins, lysed and PKD-GFP and Flag-PI4KIIIβ were precipitated using anti-GFP and anti-Flag antibodies. Samples were further processed as described in Methods. (d) Knock-down of PKD1 or PKD2 isoenzymes inhibits Flag-PI4KIIIβ phosphorylation. HEK293E cells were transfected with pSUPER-PKD1-RNAi (2 different sequences; Seq.1 or Seq.2), pSUPER-PKD2-RNAi or pSUPER-PKD3-RNAi. Flag-PI4KIIIβ was precipitated with anti-Flag M2 antibodies and analyzed for phosphorylation at Ser294 (anti-PKD pMOTIF). The blot was re-probed against Flag-PI4KIIIβ. Silencing of endogenous PKD1/2 or PKD3 was monitored by immunoblot analysis with anti-PKD antibodies. Detection of Actin served as a loading control. (e) A PKD mutant with silent mutations in the RNAi-targeted sequence rescues PKD-mediated PI4KIIIβ phosphorylation. HEK293E cells were transfected with pSUPER (control) or pSUPER-PKD1-RNAi. After 24hr cells were transfected with Flag-PI4KIIIβ, combined with PKD1.WT (wild-type) or a PKD1 mutant with two silent mutations in the PKD1-RNAi-targeted sequence (PKD1.MUT). Flag-PI4KIIIβ was precipitated with anti-Flag M2 antibodies and analyzed for phosphorylation at Ser294 (anti-PKD pMOTIF). The blot was re-probed against Flag-PI4KIIIβ. Silencing of endogenous PKD1 was monitored by immunoblot analysis with anti-PKD antibodies. Detection of Actin served as a loading control. (f) A phospho-antibody specific for pS294 in PI4KIIIβ detects endogenous PI4KIIIβ. Endogenous PI4KIIIβ was precipitated from HEK293 cells either with the polyclonal PI4KIIIβ antibody or the polyclonal pS294 serum and detected with the polyclonal pS294 serum. The blot was reprobed with a monoclonal PI4KIIIβ antibody. The preserum served as a control.