Figure 2.
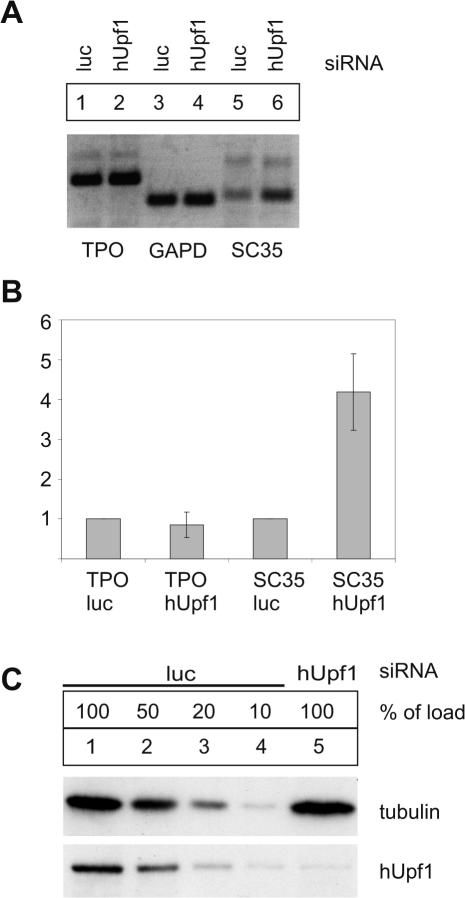
Endogenous full-length TPO mRNA is NMD resistant. (A) RT–PCR analysis of endogenous HuH7 TPO after hUpf1 depletion. Cells were transfected with siRNAs specific for hUpf1 to deplete endogenous hUpf1 protein (cDNA used in lanes 2, 4, 6). Cells transfected with siRNAs directed against luciferase (cDNA used in lanes 1, 3, 5) served as negative controls. Cells were harvested 72 h after siRNA transfection. After first strand cDNA synthesis, PCR with gene specific primers for TPO (lanes 1 and 2), GAPD (lanes 3 and 4) and SC35 (lanes 5 and 6) were performed using cDNA from luciferase- (lanes 1, 3, 5) or hUpf1-siRNA transfected cells (lanes 2, 4, 6). (B) Quantitative RT–PCR analysis of endogenous HuH7 TPO after hUpf1 depletion. LightCycler™ analysis was performed to determine the expression levels of TPO, GAPD and SC35 in cells transfected with hUpf1 siRNAs compared to luciferase-treated cells. cDNAs from the first strand synthesis described in (A) was used for LightCycler analysis. All expression levels were normalized to GAPD expression levels. NMD-sensitive SC35 served as positive control for the inhibition of NMD. SC 35 primers specifically amplify the spliced 3′-UTR with two splice variants (1,6 and 1,7 kb) as described previously (33). The level of up-modulation and standard deviations are indicated. Values and standard errors were calculated from five independent experiments. (C) Immunoblot analysis of protein lysates from HuH7 cells transfected with siRNAs against luciferase as a negative control (luc, lanes 1–4) or hUpf1 (lane 5) using a hUpf1 specific antibody. Dilutions corresponding to 50, 20 or 10% (lanes 2–4) of the initial protein amount (lane 1) from luciferase-siRNA transfected cells were loaded to assess the efficiency of the hUpf1 depletion. Reprobing with a tubulin specific antibody was performed as a loading control.