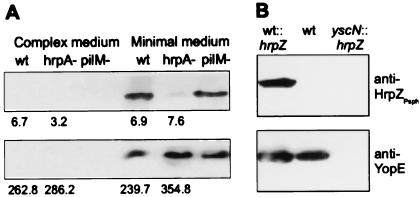
Figure 2.
Analysis of HrpZPsph secretion by Psph and by Y. enterocolitica. (A) hrp-dependent secretion of HrpZPsph. Psph race-6 wild-type (wt), race-6 hrpA− mutant (hrpA−), and a type IV pili mutant of strain HB10Y (pilM−) were grown in complex media or hrp-inducing minimal media (8). Bacteria were pelleted by centrifugation, and secreted proteins were precipitated from the culture supernatant by 5% (vol/vol) trichloroacetic acid. Proteins prepared from the supernatant (Upper) and pellet (Lower) were analyzed by SDS/PAGE and immunoblotting by using an antiserum raised against recombinant HrpZPsph (1:5,000 dilution). Numbers below individual lanes represent β-glucuronidase activity (nmol 4-methylumbelliferone released per minute per bacterium × 1010), which was determined in uidA-transformed Psph strains grown in complex and minimal growth medium (30). (B) Type III-dependent secretion of HrpZPsph. Y. enterocolitica wild-type strain KNG22703(pYV227) (wt), this strain carrying pBluescript SK(+)-hrpZ (wt∷hrpZ), and Y. enterocolitica type III secretion-deficient yscN- mutant KNG22703(pYV2276) transformed with plasmid pBluescript SK(+)-hrpZ (yscN∷hrpZ) were grown under permissive conditions. Analysis of secreted proteins was performed by using antisera raised against HrpZPsph or YopE, respectively.