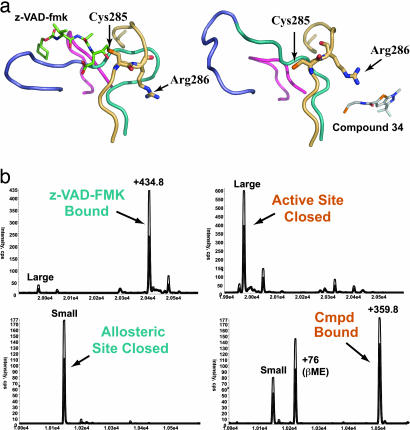
Fig. 2.
Comparison of the caspase-1 active site in active- and allosteric-site-bound structures. (a) Four loop regions that change position between the active- and allosteric site-bound conformations are shown as ribbons. The active-site residue Cys-285 and the adjacent residue Arg-286 are displayed as sticks. (Left) The active-site-bound structure is shown in complex with the active-site inhibitor z-VAD-FMK (green sticks). (Right) The allosteric-site-bound structure in complex with the allosteric inhibitor Compound 34. (b) Mass-spectrometry results of competitive labeling of the active and allosteric sites with z-VAD-FMK and Compound 34 to large and small subunits, respectively. Either inhibitor was added first to the enzyme for 30 min, followed by addition of the second inhibitor and a 30-min incubation.