FIGURE 2.
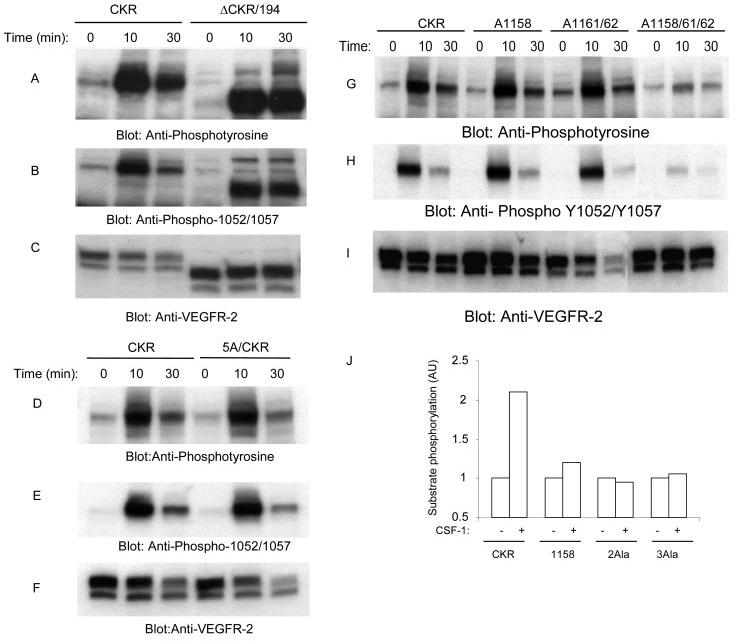
Effect of mutation of leucines 1158, 1161, and 1162 to ligand-dependent tyrosine autophosphorylation of VEGFR-2 and its ability to phosphorylate exogenous substrate. An equal number of serum-starved PAE cells expressing wild type chimeric VEGFR-2 (CKR), carboxyl-terminal deleted, ΔCKR/194, and 5A/CKR were either not stimulated or stimulated for 10 or 30 min with CSF-1 (40 ng/ml). Cells were lysed and total cell lysates were subjected to Western blot analysis using anti-phosphotyrosine antibody (A and D) or a phospho-specific 1052/1057 VEGFR-2 antibody (B and E). The same cell lysates were subjected to Western blot analysis using anti-VEGFR-2 for protein loading as a control (C and F). In a similar manner, an equal number of serum-starved PAE cells expressing wild type chimeric VEGFR-2 (CKR), A1158/CKR, A1161/1162/CKR, and A1158/1161/1162 were either not stimulated or stimulated for 10 or 30 min with CSF-1. Cells were lysed and total cell lysates were subjected to Western blot analysis using anti-phosphotyrosine antibody (G) or a phospho-specific 1052/1057 VEGFR-2 antibody (H). The same cell lysates were subjected to Western blot analysis using anti-VEGFR-2 for protein loading as a control (I). Serum-starved PAE cells expressing wild type chimeric VEGFR-2 (CKR), A1158/CKR (1158), A1161/1162/CKR (2Ala), and A1158/1161/1162/CKR (3Ala) were either not stimulated or stimulated for 10 min with CSF-1. Cells were lysed, and proteins were immunoprecipitated with anti-VEGFR-2 antibody. The immunoprecipitated proteins were extensively washed and subjected to an in vitro kinase assay as described under “Materials and Methods,” and phosphorylation of exogenous substrate was measured (J).
