FIGURE 3.
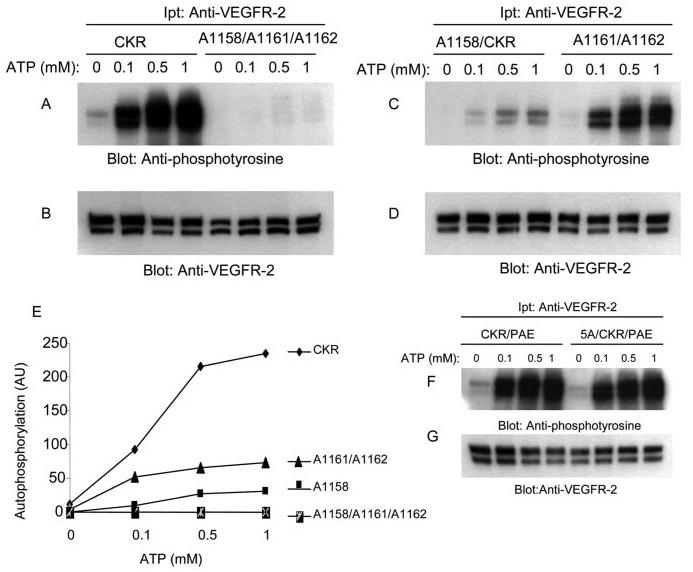
Effect of mutation of leucines 1158, 1161, and 1162 on ATP-induced tyrosine autophosphorylation of VEGFR-2. An equal number of serum-starved PAE cells expressing wild type chimeric VEGFR-2 (CKR), A1158/1161/1162, A1158/CKR, and A1161/1162/CKR were lysed without stimulation. CKR proteins were immunoprecipitated (Ipt) with anti-VEGFR-2 antibody and divided into two groups. One group was subjected to the in vitro kinase assay using ATP (A, C, and E), the second group was subjected to Western blot analysis using anti-VEGFR-2 antibody as a control for protein levels (B, D, and F). In vitro kinase assay was performed as described under “Materials and Methods.” Briefly, the immunoprecipitated proteins were incubated with different concentrations of ATP. After a 15-min incubation, the reaction was terminated by adding 2× sample buffer. The samples were boiled and subjected to Western blot analysis using anti-phosphotyrosine antibody (A, C, and E).
