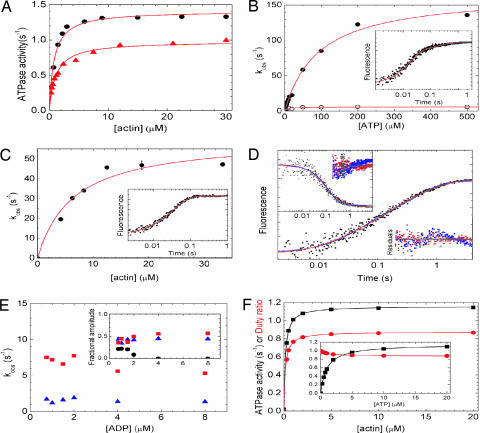
Fig. 4.
Kinetic properties of DmVIIa. (A) Steady-state actin-activated ATPase activity of DmVIIa–S1 and DmVIIa–HMM–Zipper. Hyperbolic fit to the data set yielded a maximal ATPase (Vmax) of 1.0 ± 0.1 s−1 with half-saturation (KATPase) at 1.1 ± 0.10 μM actin for DmVIIa–S1 (triangles) and Vmax = 1.4 ± 0.0 s−1 with a KATPase of 0.8 ± 0.08 μM actin for DmVIIa–HMM–Zipper (circles). (B) Observed rate constants (kobs) of the fast (filled symbols) and slow (open symbols) phases of ATP-induced dissociation of pyrene-acto-DmVIIa–S1 as measured by stopped-flow. The hyperbolic dependence of kobs on [ATP] indicated maximal kobs values of 167 and 5.9 s−1 for the fast and slow phases, respectively, in the experiment shown. Half-saturation occurred at 93 μM ATP for the fast phase. (B Inset) Trace at 50 μM ATP with kobs values of 59 s−1 (fractional amplitude: 80%) and 5.5 s−1 (20%). (C) Observed rate constants (kobs) of Pi release in double-mixing stopped-flow experiments in which mVIIa–S1 was first mixed with substoichiometric amounts of ATP, incubated for 5 s to obtain the DmVIIa–S1–products complex, and then rapidly mixed with various actin concentrations. Pi release was monitored by MDCC-PBP fluorescence (24). The kobs values showed a hyperbolic dependence on actin concentration with a maximum at 61 s−1 and half-saturation occurring at 6.4 μM actin. (C Inset) Trace at 15 μM actin with a single-exponential kobs of 30 s−1. (D) Kinetics of ADP release from acto-DmVIIa–S1. Shown is a pyrene–actin fluorescence trace obtained on mixing 0.5 μM pyrene-acto-DmVIIa–S1 plus 1 μM ADP with 100 μM ATP in the stopped-flow. A triple-exponential fit (red) to the transient shown yielded kobs of 50.1 (22% fractional amplitude), 7.2 s−1 (44%), and 1.2 s−1 (34%). The fastest phase represented ATP-induced dissociation of the nucleotide-free fraction of acto-DmVIIa–S1, and the later two phases originated from two populations of acto-DmVIIa–S1–ADP (compare Fig. 5). (D Inset) A 2′-deoxy-mant-ADP (dmADP) fluorescence transient recorded upon mixing a premixture of 1 μM actin/0.5 μM DmVIIa–S1 plus 5 μM dmADP with 1 mM ATP in the stopped-flow. Similarly to the pyrene–actin fluorescence records, biphasic ADP release was observed, and a double-exponential fit (red) yielded rate constants of 10.6 s−1 (67% fractional amplitude) and 1.3 s−1 (33%). Fits for monophasic ADP release (blue, double-exponential fit in main graph, single exponential in Inset) showed clear systematic deviations from the experimental data (see residual plots; red, biphasic ADP release; blue, monophasic ADP release) in all experiments. Time axes are the same for the data fits and the corresponding residual plots. (E) [ADP] dependence of kobs (main graph) and fractional amplitudes (Inset) of the first (black circles), second (red squares), and third (blue triangles) phase of the transients obtained in experiments described in D. [kobs values of the first phase are not related to ADP release (see above) and are omitted for clarity.] (F) Steady-state ATPase activity (black) and duty ratio (red) of DmVIIa–S1 as obtained in kinetic simulations by using the experimentally determined rate constants of Fig. 5. The duty ratio is defined as the fractional steady-state occupancy of the strongly actin-bound DmVIIa–S1 states (underlined in Fig. 5). DmVIIa–S1 reaches a maximal steady-state ATPase activity of 1.16 s−1 and a maximal duty ratio of 0.88 hyperbolically with half-saturation at submicromolar actin concentrations ([ATP] was 1 mM in the simulations, in the absence of background ADP). (F Inset) DmVIIa–S1 shows a much steeper [ATP]-saturation of its steady-state ATPase activity (half-saturation at 1.0 μM ATP) than other processive myosins ([actin] was 20 μM in the simulations, in the absence of background ADP). At saturating actin and ATP concentrations and in the absence of background ADP, the fractional steady-state occupancy of the various DmVIIa–S1 states (Fig. 5) was the following: AMDclosed, 0.71; AMDopen, 0.15; AMopen, 0.0007; AMclosed, 0.0003; AM-T, 0.008; A-MT, negligible; MT, 0.11; MDP, 0.001; AMDP, 0.02. Conditions were 25°C in 10 mM Mops (pH 7.0)/2 mM MgCl2/0.1 mM EGTA/1 mM ATP/50 mM KCl (10 mM KCl in C).