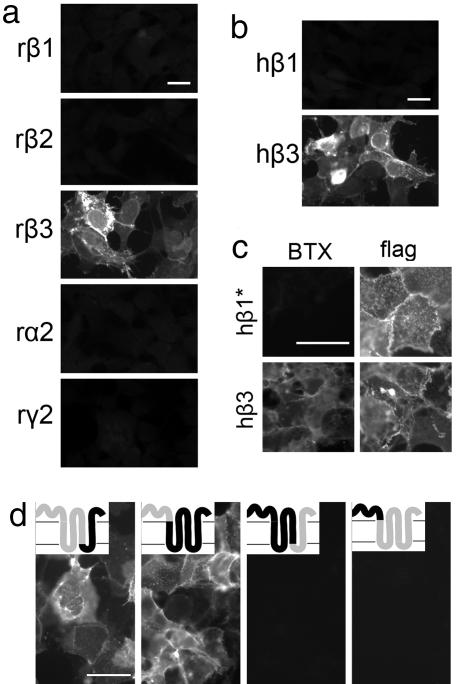
Fig. 3.
Mapping of BTX-binding sites on GABAARs. HEK 293 cells transfected with cDNAs encoding indicated subunits were stained with BTX after (a, b, and d) or before (c) fixation and permeabilization. (a) Rat (r) GABAAR β3 binds BTX, but rat GABAAR β1, β2, α2, and γ2 do not. (b) Human (h) GABAAR β3 binds BTX, but human GABAAR β1 does not. (c) A GABAAR β1 subunit that had been mutated to enhance its ability to form pentamers (β1*) failed to bind BTX. The subunit bore an amino-terminal “flag” epitope tag; antibody to the tag stained live cells, confirming that the subunit reached the cell surface. A similarly tagged wild-type β3 subunit did bind BTX. (d) Chimeric receptors containing the amino-terminal extracellular domain of GABAAR β3 fused to the remainder of GABAAR β1 bind BTX, but chimeras containing the amino-terminal extracellular domain of GABAAR β1 fused to the remainder of GABAAR β3 do not. (Insets) Portions of receptors derived from β3 (gray) and β1 (black); fusions are at amino acid 314 in the first and third chimeras and at amino acid 213 in the second and fourth chimera (11). (Scale bars, 5 μm.)