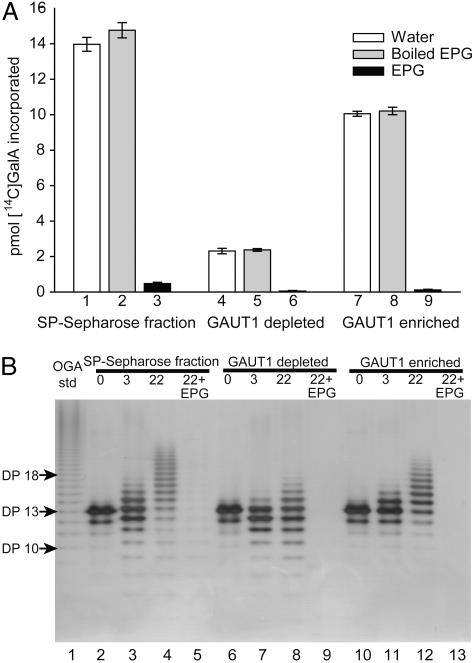
Fig. 2.
Characterization of products made by anti-GAUT1 immunoabsorbed protein. (A) Sensitivity of synthesized product to cleavage by EPG. Products synthesized during 2-h reactions containing buffer, UDP-[14C]GalA, OGA acceptors, and the Arabidopsis SP-Sepharose fraction, the SP-Sepharose fraction after immunodepletion of GAUT1 (GAUT1 depleted), or the anti-GAUT1 immunoabsorbed material (GAUT1 enriched). Each fraction was incubated overnight with water, boiled EPG, or native EPG. Radiolabeled products recovered by using the filter assay are shown. Note that small oligomers (e.g., monomer and dimer) do not bind to the filters. (B) Separation of nonradioactive GalAT reaction products by electrophoresis on a 30% polyacrylamide gel. Lanes 2–4 show the products recovered after incubation of SP-Sepharose purified solubilized Arabidopsis membrane proteins with UDP-GalA and OGAs enriched for a DP 13 for 0, 3, and 22 h at 30°C. Lanes 6–8 show the same series of incubation times with the SP-Sepharose fraction after immunodepletion with GAUT1 antiserum. Lanes 10–12 show similar series with the product synthesized by GAUT1-immunoabsorbed protein from the SP-Sepharose fraction. Lanes 5, 9, and 13 show product recovered after digestion of the respective 22-h reaction products with EPG. Lane 1 shows 0.1 μg of OGA standard of DP 7–23.