Abstract
The systemic model for floral induction, dubbed florigen, was conceived in photoperiod-sensitive plants but implies, in its ultimate form, a graft-transmissible signal that, although activated by different stimuli in different flowering systems, is common to all plants. We show that SFT (SINGLE-FLOWER TRUSS), the tomato ortholog of FLOWERING LOCUS T (FT), induces flowering in day-neutral tomato and tobacco plants and is encoded by SFT. sft tomato mutant plants are late-flowering, with altered architecture and flower morphology. SFT-dependent graft-transmissible signals complement all developmental defects in sft plants and substitute for long-day stimuli in Arabidopsis, short-day stimuli in Maryland Mammoth tobacco, and light-dose requirements in tomato uniflora mutant plants. The absence of donor SFT RNA from flowering receptor shoots and the localization of the protein in leaf nuclei implicate florigen-like messages in tomato as a downstream pathway triggered by cell-autonomous SFT RNA transcripts. Flowering in tomato is synonymous with termination of the shoot apical meristems, and systemic SFT messages attenuate the growth of apical meristems before and independent of floral production. Floral enhancement by systemic SFT signals is therefore one pleiotropic effect of FT orthologs.
Keywords: florigen, grafting, perennial model, termination, universal function
The transition from vegetative to floral meristems in higher plants is programmed by the coincidence of internal and environmental signals. Experiments in a variety of plant species have shown that inductive photoperiods cause leaves to emit mobile signals dubbed florigen (1–3), which induce flowering in the shoot apical meristems (SAMs) and have been proven by graft experiments to be conserved among related species and different response types.
Several indications implied that the Arabidopsis FT (FLOWERING LOCUS T) gene provides a possible functional link between the systemic pathways and the cell-autonomous pathways to flowering. FT is a major integrator of the genetic pathways to flowering in short and long days (4, 5); it encodes a signaling factor (6, 7) and is not expressed in the SAM proper (8) but can be detected, upon induction, in shoot apices (SAPs) containing young leaves (9). Flowering is delayed in mutant ft plants (10, 11), and when FT is overexpressed, flowering occurs earlier with a determinate inflorescence (12, 13). FT is regulated by the flowering-time gene CONSTANS in both long- and short-day plants (14, 15), and grafting experiments in Arabidopsis have shown that systemic induction of flowering by CONSTANS is most likely mediated by FT (16, 17). It was recently shown that a small fraction of heat-shock-induced FT RNA, originating in a single leaf, is found in the SAPs, suggesting that the FT mRNA itself may represent a major component of florigen (18).
We chose tomato, a photoperiod-insensitive plant, to test the premise that orthologs of the Arabidopsis FT gene can initiate a conserved, long-distance, flower-promoting pathway in diverse flowering systems. The generality of the florigen hypothesis was supported by interspecies grafting experiments (2). Grafting results are independent of the validity of promoters, the resolution of in situ hybridization patterns, inferences derived from the activation of upstream genes, or interpretations of clonal analysis. The perennial habit; the compound shoots, which permit the analysis of multiple vegetative/floral transition events in one plant (19); and the ease of grafting render tomato as a useful experimental platform for investigating the nature of florigen. We expanded the analysis in tomato with parallel experiments in long-day Arabidopsis and short-day tobacco.
The primary shoot of tomato is terminated by an inflorescence, after which the apparent main axis consists of an upright array of reiterated axillary branches called sympodial units (SUs). Each SU arises from the most proximal axillary bud of the preceding unit and consists of three vegetative nodes and a terminal inflorescence (Fig. 1A). The distinction between the primary and compound sections of sympodial plants provides two basic criteria for flowering time: the number of leaves to the first inflorescence in the primary shoot and the number of leaves between inflorescences in the compound part. Here, we identify the tomato FT ortholog as SINGLE FLOWER TRUSS (SFT), a gene regulating primary shoot flowering time, sympodial habit, and flower morphology. All aspects of the sft phenotype were complemented by graft-transmissible SFT signals, suggesting that all are the consequence of a common flowering-time defect. Significantly, graft-transmissible SFT signals substituted for light dose and two inductive photoperiodic stimuli in different species as well.
Fig. 1.
The tomato FT gene is mutated in the late-flowering sft mutant and induces premature flowering in day-neutral tomato and tobacco. (A) The primary shoot of tomato is terminated by a determinate inflorescence (I-1) after 8–12 leaves. The apparent main axis then consists of a reiterated array of SUs, each arising from the most proximal axillary bud of the preceding one and consisting of three vegetative nodes (numbered) and a terminal inflorescence (marked I-2 and I-3). (B) Enlarged view of a SU. Note that the SU unites with the basal part of leaf that subtends it (no. 3), thus placing it above the inflorescence and, in addition, displacing the inflorescence (Inf) sideways. (C) In the sft mutant shoot, the first terminal inflorescence is formed after 15–20 leaves and, in addition, delays the release of the prospective SU, thus maintaining its own pole position. The sft VI is initiated with a single flower and is subsequently composed of a mix of single flowers (∗) and leaves (L). (Inset) sft flower with its enlarged leafy sepal. (D) WT plant of a day-neutral tobacco (Samsun) flowers after 26–28 leaves. (E) Samsun plants expressing the 35S:SFT flower after only four to six leaves. (F) 35S:SFT#3 tomato plants flower after three leaves (numbered), display early release of lateral shoots (LS), and form thinner stems and smaller simpler leaves. (G) Primary precocious termination with a single terminal flower (TF) in 35S:SFT#2 is accompanied by the formation of a simple leaf (SL) and a delayed release of the first sympodial bud (arrow). The circle marks a delayed axillary bud. (H). Increasing SFT RNA levels are correlated with earlier flowering in progenies of 35S:SFT#3/+ plants. Number of leaves to flowering is indicated above each lane of the Northern blot. (Scale bars: A, D, E, and F, 10 cm; B, C, and G, 2 cm.)
Results
The Tomato Ortholog of FT Is Disrupted in Late-Flowering sft Mutants.
The putative tomato ortholog of FT, SP3D, was mapped to subsection IL3-2/3 of the tomato map (20). Our restriction fragment length polymorphism mapping suggested that sft, a late-flowering morphogenetic mutant, which was previously localized to chromosome 3 (21), also maps to this subchromosomal section. In a large-scale mutant screen involving 13,000 M2 families (22), the three most extreme late-flowering mutations displayed the sft morphogenetic syndrome (23) and were shown by complementation test to be allelic with sft (LA2460) and with each other. An additional sft allele, sft-k, was a gift from M. Koornneef (Wageningen University, Wageningen, The Netherlands).
All five sft alleles were subjected to sequence analysis, and four had lesions in the coding region of SP3D, suggesting that sft is mutated in the gene encoding the tomato FT ortholog. sft-4537 and sft-4781 have the same T-to-I missense mutation. sft-7187 has a 2-nt deletion truncating the C terminus, and the Y160 codon is deleted in sft-k (Fig. 6, which is published as supporting information on the PNAS web site). We could not identify the mutational lesion in the sft allele of the LA2460 line.
All mutant alleles had phenotypes identical to that described for sft in line LA2460 (23), and further analysis was carried out with the sft-k allele. The primary shoots of sft plants produce an inflorescence after 15–20 leaves, compared with the 8–12 leaves of its WT siblings. Apart from their late-flowering phenotype, sft plants also have an indeterminate vegetative inflorescence shoot (VI) that produces mostly leaves but also a few flowers each with a single enlarged sepal. Unlike in the WT tomato, the terminal sft VI exerts partial apical dominance over the presumptive sympodial bud, thus maintaining its own pole position (see ref. 19 and Figs. 1 B and C and 6).
SFT Induces Early Flowering in Day-Neutral Plants.
To ascertain whether SFT and FT promote early flowering in day-neutral plants, both were overexpressed in tobacco and tomato under the constitutive 35S promoter. Both transgenes induced extreme early flowering in tobacco and tomato (Fig. 1 D–G). Progeny of five 35S:SFT and 35S:FT tobacco Samsun lines flowered after producing 4–6 leaves compared with the 24–28 leaves of their nontransgenic siblings (Fig. 1 D and E; only 35S:SFT transgenic plants are shown), suggesting functional orthology in day-neutral plants. The first inflorescence arose after 3–5 leaves in the progeny of nine independent 35S:SFT tomato lines, compared with 10–12 leaves of their siblings. Homozygous plants of a weaker line, 35S:SFT#3, flowered after three leaves, but subsequent SUs maintained the regular three-nodal sympodial size (Fig. 1F). Strikingly, in the stronger 35S:SFT#2 plants (35S:SFT hereafter), the primary SAM was often arrested after forming only one or two leaves and an additional single flower. Growth is later resumed from a delayed axillary bud (circled in Fig. 1G). Notably, the sympodial growth pattern was maintained, but instead of the three leaves per SU, there were two. Decreasing flowering time in the progeny of 35S:SFT#3 was correlated with high SFT transcript levels (Fig. 1H). All early flowering transgenic plants had fewer leaflets per compound leaf, shorter internodes, and much thinner stems.
Graft Transmissible SFT Signals Complement All Developmental Defects of sft Mutant Plants.
sft mutant plants expressing the 35S:SFT transgene are indistinguishable from 35S:SFT#3 plants (Fig. 1F), confirming that sft is encoded by the tomato ortholog of FT.
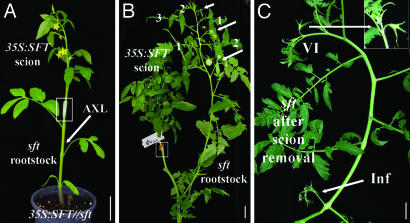
If the function of the tomato FT gene is mediated by systemic signals, it is expected that a 35S:SFT donor will rescue the WT phenotype of grafted sft receptor shoots (see Fig. 2A for a demonstration graft). Consistent with these expectations, all 19 reciprocal grafts between sft and 35S:SFT plants produced sft receptor shoots with normal flowers, normal inflorescences, and normal sympodial architecture 3–5 weeks after grafting (Fig. 2 B and C). Thus, graft-transmissible flowering signals initiated by the SFT gene rescue flowering time and morphogenetic defects in sft mutant plants.
Fig. 2.
Continuous SFT-stimulated systemic signals rescue flowering and morphogenetic defects of sft. (A) A demonstration graft. A donor 35S:SFT is grafted (boxed) onto a receptor sft rootstock with dormant axillary buds (AXL). Flowering response is followed in the released lateral shoots of the rootstock. (B) Complementation of sft by graft-transmissible 35S:SFT-stimulated systemic signals. A receptor sft shoot (right) with three successive normal SUs, each terminated by a regular inflorescence (arrows), is shown. Leaves of the rescued SUs are numbered. (C) Reversion of a rescued sft shoot after removal of the 35S:SFT donor. The last normal inflorescence (Inf) and the subsequent sft VI (Inset) are shown. (Scale bars: 3 cm.)
In contrast, reciprocal grafts involving 36 sft receptors and 22 sft donors, or, significantly, 14 WT donors, failed to complement the sft phenotype. We attribute this failure to the consumption of endogenous SFT signals in WT plants and thus to the consequent transmission of lower proportions of promoting relative to inhibiting signals.
The rescue of receptor sft in grafts required persistent emission of systemic SFT signals: Formation of normal SUs, inflorescences, and flowers in receptor sft shoots continued only as long as the 35S:SFT donor was present (Fig. 2C). Once eliminated, all mutant sft features reappeared. Likewise, branches of rescued sft scions did not continue to form normal shoots upon cutting and rerooting, and seeds borne by fruits on rescued sft receptor shoots gave rise to sft plants. Complementation of sft by means of graft junction and its reversion after the elimination of donor shoots suggests that SFT-dependent autoregulation loops are not obligatory components of the systemic regulation of flowering in the day-neutral tomato. It is possible, of course, that such circuits function redundantly: for example, by SFT activating or suppressing a member of the family or, for that matter, any other relevant gene.
Systemic SFT Signals Substitute for High Irradiance in uniflora (uf) Mutant Tomato Plants.
Tomato plants are insensitive to day length but are responsive to light intensity (24). A particularly extreme case of light-dose-dependent flowering is conditioned by the recessive uf gene. In addition, uf inflorescences are indeterminate and mostly leafy with rare replacements of a leaf by a solitary flower, providing a “pseudoshoot” (PS) (25). To examine whether SFT signals can substitute for light-dosage requirements, we used light conditions (70–150 μmol−2·s−1 at 18–24°C) under which one-third of uf plants never flower and the rest form the first and sometimes only floral PS after 30–50 leaves. Under the same conditions, uf 35S:SFT plants flowered after three leaves, and more flowers were produced along the VI (Fig. 3A and B). Similarly, in 58 reciprocal grafts with 35S:SFT donors, all uf receptor shoots (67 lateral shoots of 32 uf receptor stocks and 26 shoots of uf receptor scions) flowered early, after four to nine leaves, and formed PSs with more flowers replacing leaves (Fig. 3C). As in sft grafts, removing 35S:SFT donor shoots resulted in reversion to the uf phenotype, and early flowering was never observed in controls with WT donors or uf homografts. Thus, graft-transmissible signals stimulated by 35S:SFT substituted for the high light dose and converted leaf primordia in the PS to flowers. We also grafted 11 uf scions onto 35S:SFT rootstocks from which cotyledons, leaves, and axillary buds were eliminated. Under these circumstances, none flowered earlier.
Fig. 3.
Long-range SFT signals substitute for three distinct environmental stimuli. (A) A typical late-flowering uf plant grown under low irradiance. (Inset) A PS with a rare flower. (B) A uf 35S:SFT plant grown under low-irradiance conditions. The primary shoot is terminated by a flowering PS after three leaves. (C) Floral induction in shade-grown 35S:SFT//uf grafts (boxed). uf lateral shoots of the receptor stock formed the first flowering PS (arrow) after only seven leaves. (D) An ever-vegetative MM plant grown under long (18-h light/6-h dark) days. (E) Early flowering in long-day grown MM plants expressing the 35S:SFT transgene. (F) Tomato 35S:SFT donor scions grafted onto leaf petioles induces flowering in MM under long-day conditions. (G and H) Transactivation of ER-GFP by means of the BLS promoter as detected by CLSM before (G) and after (H) flowering. Earliest ER-GFP expression is detected in the P5 primordium but not in the SAM, flowers (F), or the inflorescence meristem (IM) of Arabidopsis apices. (I) Arabidopsis plants expressing BLS:SFT and grown under short days flowered after only 6 rather than 19 leaves. (Scale bars: A and C–F, 10 cm; B and I, 2 cm; G and H, 50 μm.)
Systemic SFT Signals Substitute for Short- and Long-Day Flowering Stimuli.
Florigen-like systemic signals are expected to substitute for different environmental stimuli in diverse plant species. In a seminal discovery, Garner and Allard (26, 27) identified a recessive mutation in the Maryland Mammoth (MM) tobacco strain that confers a short-day response on day-neutral tobacco plants. When grown under long days, cv. Samsun plants flower after 24–25 leaves (Fig. 1D), whereas MM plants do not flower at all (Fig. 3D). The tomato 35S:SFT transgene induced early flowering in both MM plants under long days and in day-neutral Samsun plants under all conditions (Figs. 3E and 1E). To see whether the SFT systemic pathway operating in tomato is also conserved in the short-day tobacco, we grafted 35S:SFT donor shoots onto leaf petioles of MM plants grown under 18-h light/6-h dark conditions. The receptor MM plants flowered 3–4 weeks after grafting, indicating that 35S:SFT signals, generated in tomato and transmitted by means of tobacco leaf petioles, induce flowering in MM apices under conditions in which they otherwise never flower (Fig. 3F).
To examine the potential of the tomato SFT signals to substitute for the long-day flowering stimulus, we studied transgenic Arabidopsis plants expressing the SFT gene under a leaf-specific promoter. We identified the BLS (At3G49950) promoter as driving expression in primordial and young leaves of the SAP of Arabidopsis but not in the SAM proper (Fig. 3 G and H). Under short days (10-h light/14-h dark), progenies of three independent BLS:SFT Arabidopsis plants flowered after only 5–7 leaves, compared with 15–19 leaves in WT plants (Fig. 3I).
Expression of SFT in Tomato Leaves Is Developmentally Regulated.
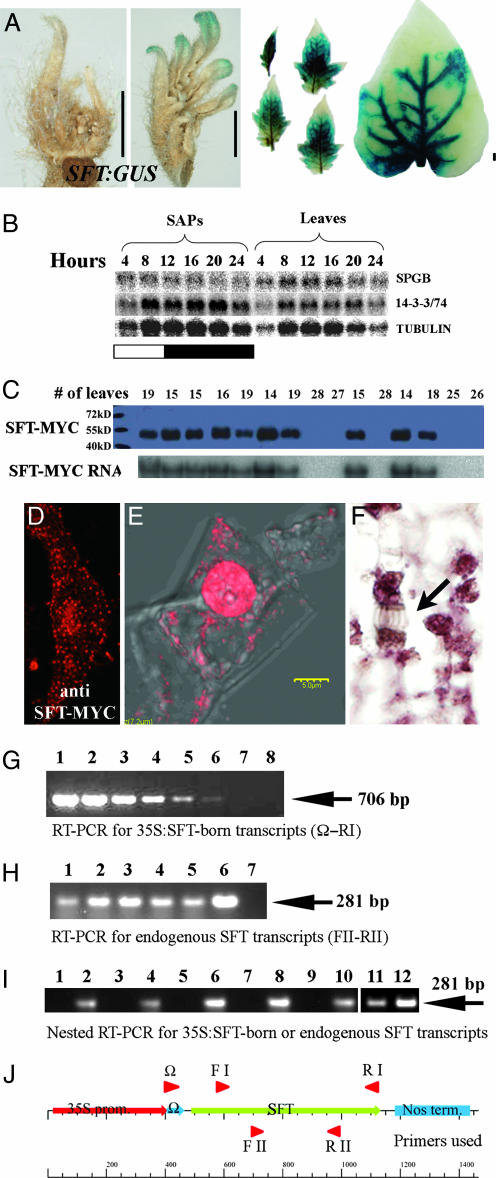
Semiquantitative RT-PCR experiments showed that SFT is normally expressed in leaves, stems, SAPs, and flowers but not in roots (20). To relate the expression pattern of SFT to its systemic functions, we examined the expression of a β-glucuronidase (GUS) reporter gene driven by a 2.3-kb upstream region of the SFT gene. A dynamic pattern of SFT:GUS staining was initiated in the growing tips of expanding leaf primordia and later in developing terminal and lateral leaflets of expanding leaves. However, no staining was detected in the SAM itself, where floral transition takes place (Fig. 4A). Concomitant with maturation, staining in the leaves advanced in a basipetal direction while distal regions lost staining. Within leaves, most staining was found in primary and secondary veins. Flowers, sepals, and petals were also stained. The staining pattern in leaves agrees closely with the polar patterns of cell proliferation that are associated with lamina expansion in tobacco and Arabidopsis (28, 29). The dynamic, temporal, and spatial expression of SFT in leaves and the observations that the removal of young leaves enhances flowering (30) and overexpression of SFT/FT in roots is insufficient to stimulate systemic flowering (31) indicate that the source, mobility, distribution, and targets of SFT signals in tomato are tightly regulated.
Fig. 4.
Cellular localization of SFT, organ distribution of SFT-interacting proteins, and SFT transcript assay in receptor graft tissues. (A) Expression profile of a GUS-tagged SFT promoter. Shown are a SAP (Left), the youngest leaf with detected expression (Center), and a series of developing leaves (Right). (Scale bars: 1 mm.) (B) Diurnal RNA expression of SFT-interacting proteins in 3- to 4-cm leaves and SAPs of tomato. (C) Correlation between SFT-MYC antigen (Upper) and SFT-MYC RNA (Lower) in progenies of a 35S:SFT-MYC/+ Samsun plant segregating for flowering time. (D–F) The SFT-MYC antigen is localized primarily in the nucleus. (D) Intracellular localization of SFT-MYC antigen in a sepal of 35S:SFT-MYC tobacco (rhodamine staining). (E and F) An optical confocal section and Nomarski image (alkaline phosphatase), respectively, of tobacco cells expressing the SFT-MYC antigen. The arrow points to the spindle of a decorated dividing cell. (G) Calibration of RT-PCR detection levels of 35S:SFT-born transcripts (Ω and RI primers) in RNA from leaves of a 35S:SFT donor and a rescued sft-k receptor. Lanes 1–6, 35S:SFT donor RNA template in 1/5 dilution series starting with 5 μg of RNA; lanes 7 and 8, 5 μg of template RNA from WT and rescued sft receptor SAPs, respectively. (H) RT-PCR detection of endogenous SFT RNA in rescued receptor sft organs (5 μg of RNA; FII and RII primers). Lanes 1–4, template RNA of sft-k receptor from young leaves, SAPs, stem, and flowers, respectively; lanes 5 and 6, WT and donor 35S:SFT leaves, respectively; lane 7, no template control. (I) Nested RT-PCR detection (primers FII and RII) of 35S:SFT transcripts (odd lanes, first-round primers Ω and RI) in comparison with SFT transcripts (even lanes, first-round primers FI and RI) in the four rescued sft recipient organs as above (lanes 1–8), in WT (lanes 9 and 10), and in 35S:SFT donor (lanes 11 and 12). Dilution ratios of first-round PCR were 1/1,000 for the 35S:SFT and 1/105 for the endogenous SFT transcripts. (J) A scheme of the 35S:SFT transgene showing the primers used in the PCR experiments.
Genes Encoding Major SFT-Interacting Proteins Are Expressed in Leaves.
A G-box factor called SPGB and a specific 14-3-3 adapter protein, 14-3-3/74, have been shown to interact with the tomato SP protein, the ArabidopsisFT protein (7), and the SFT protein (unpublished data). SPGB's closest homolog in Arabidopsis is encoded by FD, and the significance of an FT–FD interaction for FT-mediated floral induction in Arabidopsis has been demonstrated (9, 31). To see whether the organ expression profiles of these interacting proteins overlap with those of SFT in leaves, the daily expression profiles of SPGB and 14-3-3/74 were monitored in 3- to 4-cm-long leaves and in SAPs collected from seedlings just before flowering. Both SPGB and 14-3-3/74 were expressed in leaves and apices throughout the day at levels exceeding that of SFT RNA (Fig. 4B). Note that SFT RNA could not be detected by Northern blot analysis in WT plants (Fig. 1H).
The SFT Antigen Resides in Nuclei, and 35S:SFT Transcripts Do Not Cross Graft Unions.
SFT and its interacting partners are coexpressed in leaves, but SFT is not expressed in the target meristems. To explore other components of the systemic pathway, we examined the cellular localization of the SFT protein and the distribution of 35S:SFT donor transcripts in flowering graft receptors. The intracellular localization of the SFT protein was determined in early flowering tobacco plants expressing the 35S:SFT-13XMYC gene (Fig. 4C). Immunostaining followed by confocal analysis of a SFT-MYC antigen indicated that in plants induced to flower by SFT, the SFT-MYC antigen is localized primarily in the nucleus, although weak cytoplasmic staining was detected as well (Fig. 4 D–F; see also corroborating evidence in Fig. 7, which is published as supporting information on the PNAS web site). Similar results using a GFP-tagged FT were also reported in Arabidopsis (31).
To test whether SFT RNA is mobile, a donor 35S:SFT scion was grafted onto a sft receptor stock. When reversion to the WT habit in the receptor sft shoots was evident in three consecutive SUs, RNA was extracted from apices, leaves, stems, and flowers of the receptor sft shoots (Fig. 2). Semiquantitative RT-PCR successfully detected 35S:SFT mRNA (distinguished by its 5′UTR Ω sequence) in 2 ng of RNA from donor leaves (Fig. 4G, lanes 1–6). No such transcript could be detected in 5 μg of RNA of the receptor or of WT control apices. In contrast, clear detection of the endogenous SFT RNA in the four receptor organs and in WT and donor leaves was evident (Fig. 4H).
For a more sensitive assay, the blank recipient RT-PCR samples were subjected to a second round of PCR using nested primers. Still no amplification products were detected in any of the receptor samples (Fig. 4I, odd lanes), whereas the same procedure detected endogenous sft RNA even after an additional 100-fold dilution (Fig. 4I, even lanes). Negative WT and positive donor RNA controls (Fig. 4I, lanes 9 and 10 and lanes 11 and 12, respectively) showed the expected absence and presence, respectively, of 35S:SFT-derived RNA.
Systemic SFT Messages Can Target Growth and Termination of Vegetative Apical Meristems.
Together with early flowering, constitutive expression of the 35S:SFT gene conferred a reduction in leaf complexity, shorter internodes, thinner stems, and arrested apices. We investigated the potential nonautonomous SFT action on the apical meristem and stem growth by driving SFT expression with organ-specific promoters. In tomato, the Arabidopsis FIL (filamentous flower) promoter drives expression throughout leaf primordia and then becomes restricted to initiating leaflets and the abaxial side of the leaves. No expression of the FIL promoter was detected within SAMs, rib meristems, or stems by using the GUS or the GFP reporters (Fig. 5A and B, respectively; see Supporting Methods, which is published as supporting information on the PNAS web site, for constructs of transgenes).
Fig. 5.
Meristem termination, a target for systemic SFT/FT signals. (A) FIL≫GUS is expressed in young tomato leaves but not in the SAM proper (Inset) or underlying stem. (B) Expression of FIL≫ER-GFP is detected in P1 leaf primordia but not in the SAM of tomato plants. (C) Early flowering, simple leaves (SL), short internodes, and inflorescences with single flowers (arrows) in the FIL≫SFT#1. (D) Early flowering with single flower, meristem arrest, and simple leaves in FIL≫FT#5 plant. The arrested primary apex consists of two leaves and a terminal flower (TF). Subsequent laterals (AXLI and AXLII) and their axillary derivatives will also form attenuated shoots with arrested apices and give rise to plants similar to that shown in C. In contrast, WT SAP (Inset) displays continuous growth upon flowering. (E) A complete arrest of the primary SAM in extreme FIL≫FT#7. The primary apex was consumed after forming three leaves. An axillary subtended by cotyledon, which consists of only one leaf, is indicated. (F) Tomato seedlings expressing the BLS:SFT transgene. Note the early flowering (upper left Inset) and the complete attenuation of the preflowering second leaf (lower left Inset). Arrows denote expression driven by transactivation. (Scale bars: A, 2 mm; B, 100 μm; C, 5 cm; D–F, 1 cm.)
As illustrated in Fig. 5 C–F, early flowering, reduced stem and leaf growth, and frequent meristem arrest were obtained in tomato transgenic plants expressing FT or SFT under the leaf-specific FIL promoter or the BLS promoter, respectively. These observations reveal the potential of FT orthologs to elicit systemic effects on growth independent of flower formation, suggesting their potential role for the long-range conditioning of meristems concomitant with floral induction.
Discussion
In its pure conceptual form, the florigen paradigm requires that a particular component of the systemic pathway be shared by all plants (2). Systemic floral-stimulating effects of some plant hormones, such as gibberellins, fulfill all of the criteria for florigen in some species but not in others (2). Likewise, the CONSTANS gene and its orthologs, which systemically induce flowering in some plants (16) but not in others (32), do not meet this criterion. FT orthologs accelerate flowering by means of endogenous expression in short- and long-day plants (14, 15), by endogenous misexpression in long-day Arabidopsis (12, 13), and, as shown here, in day-neutral tomato and tobacco and in short-day MM tobacco (Figs. 1 and 3). To deliver its messages systemically, SFT must exploit a mechanism that is common to the three species: SFT systemic signals generated in leaves were as effective as FT in replacing long days in Arabidopsis, and graft-transmissible SFT signals processed in tomato shoots effectively substituted for the short-day stimulus in MM tobacco. Taken together, the effects of SFT-stimulated signals abide by all tenets of the florigen hypothesis: long-distance induction of flowering, graft-transmissible signals conserved among different flowering systems, and signals originating in leaves but not in roots.
Components of the Florigen Pathway.
What, then, is the nature of the SFT signal, and where in the pathway from the integration of stimuli to flowering is its primary function situated? Because systemic SFT signals are equally effective in tomato, tobacco, and Arabidopsis (Figs. 2 and 3), it is unlikely that the core systemic mechanism will be different. In tomato, SFT transcripts did not cross graft unions at detectable levels (Fig. 4). In Arabidopsis, however, transgene-born RNA was detected in SAPs (including primordial leaves) shortly after heat-induction of FT in a single leaf (18).
There are the obvious differences in growth habits and in sensitivity to photoperiod between the two species. In addition, systemic flowering in tomato requires persistent emission of SFT-triggered signals (Fig. 2), and the experimental system is more robust because the source and target were separated by graft unions, by considerable distance, and by genotype. It is possible that a moving RNA in tomato is unstable or that our assay was not as sensitive. At the same time, the tomato FD homolog, SPGB, is expressed in leaves (Fig. 4), potentially making it unnecessary for SFT RNA to travel toward its interacting partners as implied for Arabidopsis (9, 31). Therefore, the analysis in tomato favors a florigen-like model in which a downstream systemic pathway is initiated by cell-autonomous functions of SFT RNA. From this perspective, the options of systemic SFT polypeptides and of amplified intercellular transduction pathways are equally plausible.
Systemic Acceleration of Flowering: A Pleiotropic Function of SFT.
Intercalary meristems of the stems and leaves were attenuated by the endogenous overexpression of SFT and by its systemic signals even before and independent of flower formation. Apical meristems were induced to form terminal inflorescences, or, alternatively, were temporarily or permanently arrested, by high levels of systemic SFT signals (Fig. 5). Thus, growth and termination are targets for florigen-compatible signals triggered by SFT. Possible mediators of such common responses are other “flowering” genes activated in leaves in response to elevated FT levels (33) or factors yet to be discovered.
Steeves and Sussex (28) speculated that the induction of flowering/termination in the vegetative SAP might be comparable with the growth determination of a leaf primordium. We are further suggesting that floral transition and growth attenuation, instead of being the consequence of one another, are two facets of the same cellular responses. This view is consistent with systemic induction of flowering being one pleiotropic effect of SFT/FTorthologs. If growth were the primary target of SFT, the systemic signals might involve conditioning of the apical meristem by means of a finely regulated temporal change in cell proliferation patterns, providing the context/time required for the vegetative-to-reproductive switch.
Materials and Methods
Plant Material.
All tomato (Solanum lycopersicon L.) strains were a gift from the C. M. Rick Center (Davis, CA). Three additional sft alleles were identified as late-flowering mutants in a screen of a tomato mutant library (22). sft-k was a kind gift from M. Koornneef, and the Arabidopsis FT clone was a gift from D. Weigel (MPI, Tübingen, Germany).
Plasmids, Constructs, and Growth Conditions.
Details of plasmid constructs and plant care used in this study can be found in Supporting Methods and Table 1, which is published as supporting information on the PNAS web site.
Grafting.
We used a classic wedge-shaped/slit grafting technique, with the site of union wrapped by parafilm and plants kept for 2–3 days in the shade and all together 7 days in 80% humidity provided by plastic bags. Success exceeded 90%.
Confocal Imaging and Microscopy.
Tissue was fixed in 2.5% paraformaldehyde overnight, osmotically adjusted, and frozen, and 20- to 45-μm sections were made with a Leica 2000 microtome (Leica, Deerfield, IL). Fluorescence was observed with a CLSM500 microscope (Olympus, Melville, NY) with argon laser excitation (488 nm; 505–525 nm for GFP; 560–590 nm for propidium iodine emission). Scanning electron microscopy analysis was carried out as described in ref. 19.
Immunohistochemistry.
For details of fixation, antibodies, and stains, see Supporting Methods.
Molecular Procedures.
Cloning, RNA extraction, and blot analysis were conducted according to established procedures.
Supplementary Material
Acknowledgments
We thank Robert Fluhr and Benny Horowitz for critical reading of the manuscript. The equal contributions of the E.L. and Y.E. laboratory members are acknowledged. This work was supported by grants from the Israel Science Foundation (ISF) and German–Israeli Project Cooperation (to Y.E.) and from the ISF, Human Frontier Science Program Organization, and the European Union (to E.L.).
Abbreviations
- FIL
filamentous flower
- FT
flowering locus T
- SFT
single flower truss
- SU
sympodial unit
- MM
Maryland Mammoth
- PS
pseudoshoot
- SAM
shoot apical meristem
- SAP
shoot apex
- VI
vegetative inflorescence shoot
- GUS
β-glucuronidase.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Chailakhyan M. K. Dokl. Akad. Nauk SSSR. 1936;13:79–83. [Google Scholar]
- 2.Zeevaart J. A. D. Annu. Rev. Plant Physiol. 1976;27:321–348. [Google Scholar]
- 3.Vince-Prue D. Photoperiodism in Plants. London: McGraw–Hill; 1975. [Google Scholar]
- 4.Boss P. K., Bastow R. M., Mylne J. S., Dean C. Plant Cell. 2004;16(Suppl):S18–S31. doi: 10.1105/tpc.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blazquez M. A., Weigel D. Nature. 2000;404:889–892. doi: 10.1038/35009125. [DOI] [PubMed] [Google Scholar]
- 6.Bradley D., Ratcliffe O., Vincent C., Carpenter R., Coen E. Science. 1997;275:80–83. doi: 10.1126/science.275.5296.80. [DOI] [PubMed] [Google Scholar]
- 7.Pnueli L., Gutfinger T., Hareven D., Ben-Naim O., Ron N., Adir N., Lifschitz E. Plant Cell. 2001;13:2687–2702. doi: 10.1105/tpc.010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takada S., Goto K. Plant Cell. 2003;15:2856–2865. doi: 10.1105/tpc.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wigge P. A., Kim M. C., Jaeger K. E., Busch W., Schmid M., Lohmann J. U., Weigel D. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Garcia L., Modueno F., Wlikinson M., Haughn G., Salinas J., Martinez-Zapater J. M. Plant Cell. 1997;9:1921–1934. doi: 10.1105/tpc.9.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koornneef M., Alonso-Blanco C., Blankestijn de-Vries H., Hanhart C. J., Peeters A. J. M. Genetics. 1998;148:885–892. doi: 10.1093/genetics/148.2.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kardailsky I., Shukla V. K., Ahn J. H., Dagenais N., Christensen S. K., Nguyen J. T., Chory J., Harrison M. J., Weigel D. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi Y., Kaya H., Goto K., Iwabuchi M., Araki T. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- 14.Samach A., Onouchi H., Gold S. E., Ditta G. S., Schwarz-Sommer Z., Yanofsky M. F., Coupland G. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- 15.Hayama R., Yokio S., Tamaki S., Yano M., Shimamoto K. Nature. 2003;422:719–722. doi: 10.1038/nature01549. [DOI] [PubMed] [Google Scholar]
- 16.An H., Roussot C., Suarez-Lopez P., Corbesier L., Vincent C., Pineiro M., Hepworth S., Mouradov A., Justin S., Turnbull C., Coupland G. Development (Cambridge, U.K.) 2004;131:3615–3626. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- 17.Ayre B. G., Turgeon R. Plant Physiol. 2004;135:2271–2278. doi: 10.1104/pp.104.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang T., Bohlenius H., Eriksson S., Parcy F., Nilsson O. Science. 2005;309:1694–1696. doi: 10.1126/science.1117768. [DOI] [PubMed] [Google Scholar]
- 19.Pnueli L., Carmel-Goren L., Hareven D., Gutfinger T., Alvarez J., Ganal M., Zamir D., Lifschitz E. Development (Cambridge, U.K.) 1998;125:1979–1989. doi: 10.1242/dev.125.11.1979. [DOI] [PubMed] [Google Scholar]
- 20.Carmel-Goren L., Liu T. S., Lifschitz E., Zamir D. Plant Mol. Biol. 2003;52:1215–1222. doi: 10.1023/b:plan.0000004333.96451.11. [DOI] [PubMed] [Google Scholar]
- 21.Kerr E. A. Rep. Tomato Genet. Coop. 1982;32:31. [Google Scholar]
- 22.Menda N., Semel Y., Peled D., Eshed Y., Zamir D. Plant J. 2004;38:861–872. doi: 10.1111/j.1365-313X.2004.02088.x. [DOI] [PubMed] [Google Scholar]
- 23.Molinero-Rosales N., Latorre A., Jamilena M., Lozano R. Planta. 2004;218:427–434. doi: 10.1007/s00425-003-1109-1. [DOI] [PubMed] [Google Scholar]
- 24.Calvert A. J. Hortic. Sci. 1959;34:154–162. [Google Scholar]
- 25.Dielen V., Kinet J.-M. Plant Growth Regul. 1998;25:149–157. [Google Scholar]
- 26.Allard H. A. Am. Nat. 1919;53:218–223. [Google Scholar]
- 27.Garner W. W., Allard H. A. J. Agric. Res. 1920;18:553–606. [Google Scholar]
- 28.Steeves T. A., Sussex I. M. Patterns in Plant Development. Cambridge, U.K.: Cambridge Univ. Press; 1989. [Google Scholar]
- 29.Donnelly P., Bonetta D., Tsukaya H., Dengler R., Dangler N. G. Dev. Biol. 1999;215:407–419. doi: 10.1006/dbio.1999.9443. [DOI] [PubMed] [Google Scholar]
- 30.Leopold A. C., Lam S. L. Proc. Am. Soc. Hortic. Sci.; 1960. pp. 543–547. [Google Scholar]
- 31.Abe M., Kobayashi Y., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Garcia J. F., Virgos-Soler A., Prat S. Proc. Natl. Acad. Sci. USA. 2002;99:15211–15216. doi: 10.1073/pnas.222390599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teper-Bamnolker P., Samach A. Plant Cell. 2005;17:2661–2675. doi: 10.1105/tpc.105.035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.