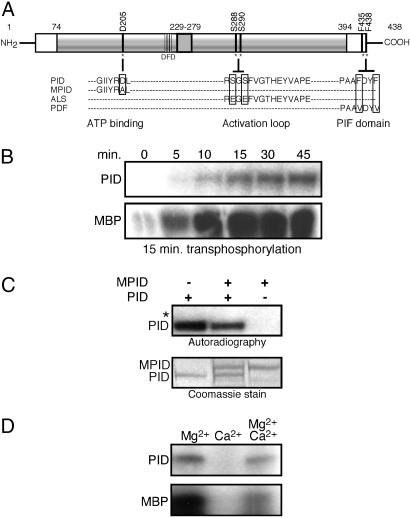
Fig. 1.
Intramolecular autophosphorylation activates PID transphosphorylation activity. (A) Schematic representation of PINOID, showing the wild-type sequence of the ATP binding domain, activation loop, and PIF domain, and the corresponding changes introduced in the MPID, ALS, and PDF derivatives. (B) Autoradiograph showing that autophosphorylation of PID is required for its transphosphorylation of MBP. (Upper) PID was incubated in kinase buffer for the indicated times. (Lower) For each time point, unlabeled, autophosphorylated PID was mixed with MBP and 5 μCi of [γ-32P]ATP (1Ci = 37 GBq) and incubated for an additional 15 min. The results shown are representative of three independent experiments. (C) PID autophosphorylates intramolecularly. His-tagged PID and GST-tagged MPID were coincubated with 10 μCi of [γ-32P]ATP and kinase buffer. The asterisk marks the location of nonphosphorylated GST-MPID visible in the Coomassie-stained gel. All autoradiographs shown are 24-h exposures. Identical results were obtained in two independent experiments. (D) Calcium inhibition of PID activity. PID activity was assayed in the presence of 15 mM MgCl2, 15 mM CaCl2, or both. For each reaction, PID was allowed to autophosphorylate (Upper) for 45 min before the addition of 3 μg of MBP (Lower). Experiments were performed in triplicate.