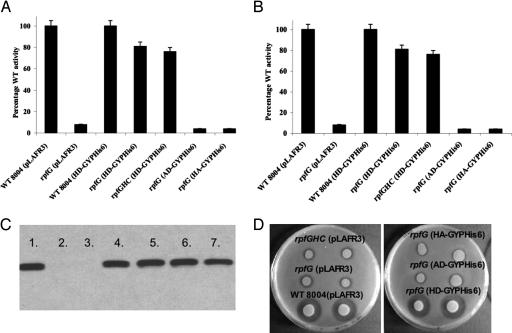
Fig. 4.
Mutation of the signature H and D residues in the HD-GYP domain abolishes its regulatory function. (A and B) Variant proteins with H231A and D232A alteration (labeled AD-GYP and HA-GYP) are unable to restore production of mannanase (A) and endoglucanase (B) to rpfG and rpfGHC mutants of Xcc. Values given are the means and standard errors of triplicate measurements. (C) Western blot analysis with an anti-His6 antiserum shows that all variant proteins are expressed in Xcc. Lanes: 1, 8004 (HD-GYPHis6); 2, 8004 (pLAFR3); 3, rpfG (pLAFR3); 4, rpfG (HD-GYPHis6); 5, rpfG (AD-GYPHis6); 6, rpfG (HA-GYPHis6); 7, rpfG (AA-GYPHis6). (D) Variant proteins H231A and D232A are unable to restore production of protease to rpfG of Xcc. The level of protease production was assessed by zones of clearing produced after growth of bacteria on skimmed milk agar plates. The H231AD232A variant is also inactive, and similar results are seen in the rpfGHC mutant background (data not shown).