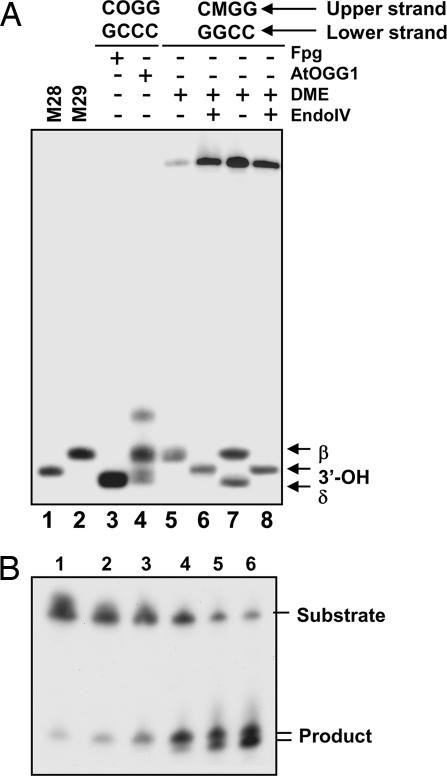
Fig. 6.
Products formed by DME upon excision of 5-meC. (A) Double-stranded oligonucleotide substrates containing a 5-meC·G (lanes 5–8) or an 8-oxoG·C pair (lanes 3 and 4) in a CpG context were incubated with purified Fpg (lane 3), AtOGG1 (lane 4), or DME (lanes 5–8), and reaction mixtures were separated in a denaturing polyacrylamide sequencing gel (40 × 20 cm). The products formed by DME were further treated with endonuclease IV (lanes 6 and 8) to analyze the nature of 3′ termini. Substrates and enzymes used are indicated at the top of the gel. Oligonucleotide markers of 28 and 29 nucleotides were loaded in lanes 1 and 2, respectively. The β- and δ-elimination products and those carrying 3′-OH termini are indicated by arrows. (B) A double-stranded oligonucleotide substrate containing a 5-meC·G pair in a CpG sequence context was incubated with purified DME, and reactions were stopped at different times. Lanes 1–6 correspond to reaction times of 0.25, 0.5, 1, 2, 10, and 24 h, respectively. Products were separated in a 12% denaturing polyacrylamide gel and visualized by autoradiography.