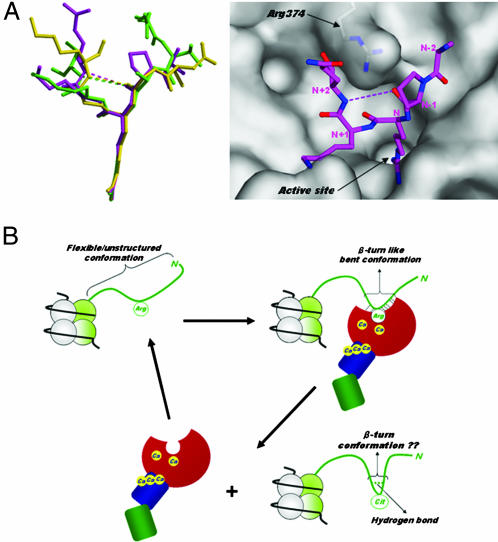
Fig. 4.
Histone N-terminal structures. (A Left) Structural comparison of PAD4-bound forms. Peptides H3-1, H3-2, and H4 are shown as ball-and-stick representations colored green, magenta, and yellow, respectively, as in Fig. 3 A–C. (A Right) Top view of the peptide H3-2 structure shown in Left, together with a molecular surface representation near the active site cleft. The weak intrapeptide interactions between the backbone oxygen at (N − 1) position and the backbone nitrogen at (N + 2) position are shown as dotted lines. (B) Possible conformational change of the histone N-terminal tail in histone citrullination. The histone N-terminal tail protruding from the nucleosome core particle is shown as green ribbon. The structure of PAD4 is drawn in the same way as in Fig. 2B.