Fig. 2. Mutation at the codon 205 of the Stk11/Lkb1 protein led to the loss of its cell growth inhibition function.
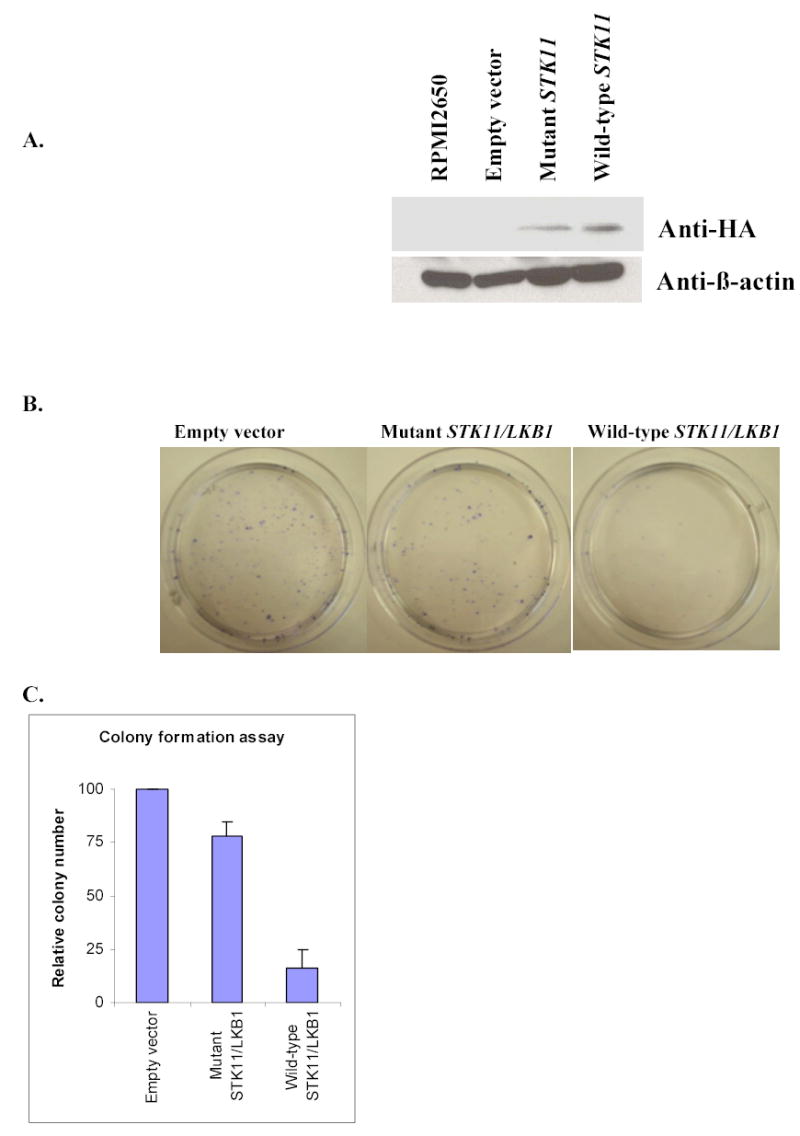
A, Western blotting analysis of RPMI 2650 cells transiently transfected with expression vectors carrying STK11/LKB1 wild-type-HA, A205T mutant-HA, or empty vector respectively. Twenty-five μg of total protein was resolved on 10%SDS-PAGE gel and transferred onto nitrocellulose membrane. Detection was performed using primary antibody mouse monoclonal anti-β-actin (Sigma) or mouse monoclonal anti-HA antibody (Roche) at 1:5000 and 1:500 respectively. The secondary antibody goat anti-mouse IgG-HRP (Sigma) was used at a final dilution of 1:5000. RPMI 2650 without transfection was loaded in first lane as an endogenous Stk11/Lkb1 protein control. Anti-β-actin bands showed that the proteins of four samples were loaded equally. Anti-HA bands showed that the ectopic protein expression level from the mutant STK11/LKB1 vector was equal to that of the wild-type in transfected RPMI 2650 cells. B, Giemsa-stained G418-resistant colonies of the RPMI 2650 cells transfected with indicated expression vectors. RPMI 2650 cells were cultured to 60–70% confluence in 10-cm dishes and transfected respectively with 1 mg of the wild-type Stk11/Lkb1, A205T mutant, or pcDNA3 empty vector using Fugene-6 transfection reagent (Roche). After 48 h transfection, G418 was added to the medium to give a final concentration of 2 mg/ml. After 18–20 day’s selection, the cells were stained with Giemsa, and the average number of the colonies present in each dish was photographed and counted. C, Relative numbers of G418-resistant colonies after transfection with indicated vectors, represented as the percentage of colonies with respect to the control (pcDNA3 empty vector). Standard deviations are derived from four independent experiments.
