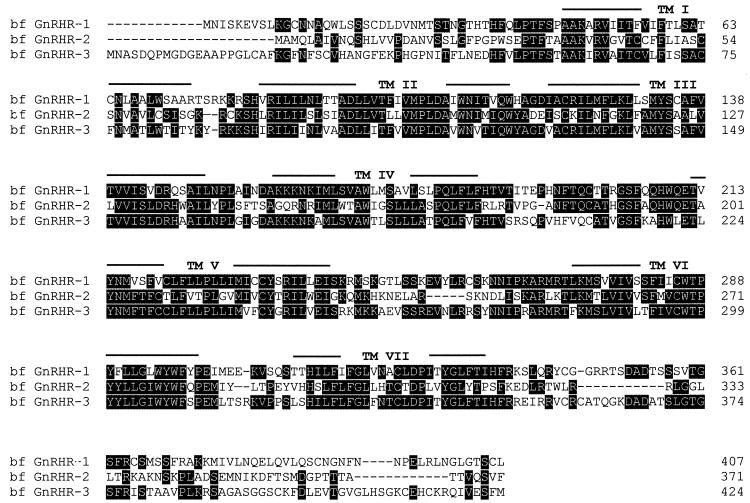
Figure 1.
Comparison of the deduced amino acid sequences of the three types of bfGnRHRs. Heavily shaded residues are identical in two or three of the receptors. Amino acid numbers are shown (Right). Gaps introduced for optimal alignment are indicated as dashes. The putative transmembrane domains are indicated (Top). BfGnRHR-1 shares 40% amino acid identity with bfGnRHR-2 and 53% with bfGnRHR-3, whereas bfGnRHR-2 shares 40% amino acid identity with bfGnRHR-3.