TABLE 1.
Association rate constants of interactions between the HIV-1 proteases and ligands
| Enzyme | Inhibitor | No.of atoms |
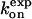 M−1s−1 M−1s−1
|
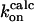 M−1s−1 M−1s−1
|
|---|---|---|---|---|
| Wild-type | Amprenavir | 70 | >5.00 × 106 | |
| Indinavir | 94 | 2.4 × 106 ± 0.3 × 106 | ||
| Saquinavir | 100 | 1.1 × 106 ± 0.2 × 106 | ||
| Substrate | ∼150 | 104–10 6 | ||
| Mutant | Amprenavir | 70 | >5.00 × 106 | >5.00 × 106 |
| L90M | Indinavir | 94 | >5.00 × 106 | ∼2.4 × 106 |
| Saquinavir | 100 | 1.7 × 106 ± 0.7 × 106 | ∼1.1 × 106 | |
| Substrate | ∼150 | n.a. | ∼104–106 | |
| Mutant | Amprenavir | 70 | 3.3 × 106 ± 0.8 × 106 | >5.00 × 106 |
| G48V | Indinavir | 94 | 2.3 × 106 ± 0.6 × 106 | ∼2.4 × 106 |
| Saquinavir | 100 | 2.1 × 106 ± 0.7 × 106 | ∼1.1 × 106 | |
| Substrate | ∼150 | n.a. | ∼104–106 | |
| Mutant | Amprenavir | 70 | 1.0 × 106 ± 0.1 × 106 | ∼106 |
| G48V/V82A/I84V/L90M | Indinavir | 94 | 7.2 × 105 ± 0.5 × 105 | ∼3.4 × 105 |
| Saquinavir | 100 | 3.3 × 105 ± 1.1 × 105 | ∼1.5 × 105 | |
| Substrate | ∼150 | n.a. | ∼103–105 |
