Abstract
The effect of cholesterol removal by methyl-β-cyclodextrin on the dipole potential, ψd, of membrane vesicles composed of natural membrane lipids extracted from the kidney and brain of eight vertebrate species was investigated using the voltage-sensitive fluorescent probe di-8-ANEPPS. Cyclodextrin treatment reduced cholesterol levels by on average 80% and this was associated with an average reduction in ψd of 50 mV. Measurements of the effect of a range of cholesterol derivatives on the ψd of DMPC lipid vesicles showed that the magnitude of the effect correlated with the component of the sterol's dipole moment perpendicular to the membrane surface. The changes in ψd observed could not be accounted for solely by the electric field originating from the sterols' dipole moments. Additional factors must arise from sterol-induced changes in lipid packing, which changes the density of dipoles in the membrane, and changes in water penetration into the membrane, which changes the effective dielectric constant of the interfacial region. In DMPC membranes, the cholesterol-induced change in ψd was biphasic, i.e., a maximum in ψd was observed at ∼35–45 mol %, after which ψd started to decrease. We suggest that this could be associated with a maximum in the strength of DMPC-cholesterol intermolecular forces at this composition.
INTRODUCTION
Cholesterol is a major component of cell membranes and constitutes up to 50% of lipid in membrane rafts (1). An important physical effect of cholesterol is to increase the membrane's internal electrical dipole potential, ψd, which is one of the major mechanisms by which it modulates ion permeability (2–5). The dipole potential is an electrical potential within lipid membranes, which arises because of the alignment of dipolar residues of the lipids and/or water dipoles in the region between the aqueous phases and the hydrocarbon-like interior of the membrane (6–8). Depending on the structure of the lipid, its magnitude can vary from ∼100 to >400 mV, positive in the membrane interior (9). Recent investigations have suggested that it affects numerous different biological membrane processes, including the conductance of the gramicidin channel (10,11), membrane insertion and folding of amphiphilic peptides (12), membrane fusion (13), phospholipase A2 activity (14), the kinetics of redox reactions at membrane surfaces (15), skin permeability (16), the activity of the Na+,K+-ATPase (17), membrane partitioning of general anesthetics (18), and the modulation of molecule-membrane interactions in lipid rafts with possible effects on cell signaling (19–21).
If cholesterol is included into a synthetic lipid membrane, there is a significant increase in the dipole potential. Szabo (2) showed that adding cholesterol to a monoolein bilayer could increase the dipole potential by up to 100 mV. Using egg phosphatidylcholine (egg PC) monolayers, McIntosh et al. (5) observed an increase in dipole potential from 415 mV in the absence of cholesterol to 493 mV at an equimolar concentration. Szabo (2) concluded that the change in dipole potential must have its origin in changes in the orientation, strength, and packing density of molecular dipoles at the membrane surface. However, he did not speculate on which molecular dipoles were involved. McIntosh et al. (5), on the other hand, argued that if cholesterol increases the area per lipid molecule in a monolayer, the increase in dipole potential cannot originate from the phospholipid molecules and they attributed it to a cholesterol-induced reorganization of the interfacial water. They appear to have ignored the possibility that the cholesterol molecule itself could be increasing the dipole potential via its own dipole moment.
In principle there are two ways in which a cholesterol-induced change in the dipole potential could modulate the passive ion permeability through the lipid phase of a membrane. One is via a change in the activation energy for ion transport. The other is via a change in the preexponential factor of the Arrhenius equation, which is related to the frequency of collision of the ion with one side of the membrane before its diffusion across it. Since the major energy barrier for ion diffusion is likely to be in the hydrophobic interior of the membrane whereas the dipole potential drops across the headgroup region of the membrane, Krull and co-workers (3,4) have suggested that the major effect of the dipole potential is likely to be via the preexponential factor, perhaps through an effect on the partition coefficient of ions between the aqueous phase and ion-binding sites located within the membrane but close to the aqueous interface. This effect would be described by a Boltzmann distribution, yielding an exponential dependence of the ion's membrane/water partition coefficient on the dipole potential.
Since the early 1990s few experimental studies of the effects of cholesterol and its derivatives on the dipole potential have been carried out. The likely reason for this is that there is no electrical method of directly experimentally determining its value. All methods involve indirect methods, e.g., measurements of the ratio of conductance of hydrophobic anions and cations (6,22,23) and electrical monolayer measurements (24,25). Because of the difficulty of experimental determination and encouraged by improvements in computer capability, this has, therefore, become a fertile playground for computer simulations, in particular molecular dynamics (MD) simulations. Up to now, however, to our knowledge, no general agreement has been reached on the mechanism by which cholesterol modifies the dipole potential. Surprisingly, one MD study even predicted that the addition of 50 mol % cholesterol to a dimyristoylphosphatidylcholine (DMPC) bilayer should decrease the dipole potential to below half that of a pure DMPC bilayer (26), i.e., the opposite of experimental observation. According to a recent MD study by Hofsäss et al. (27), the contribution of cholesterol itself to the dipole potential is negligible because of its small dipole moment in comparison to that of DPPC. But they point out that the contribution from the lipid headgroup depends strongly on its tilt. Smondyrev and Berkowitz (28), on the other hand, found in their MD calculations a significant effect of the cholesterol molecule's dipole moment on the dipole potential and an even stronger effect for 6-ketocholestanol. Chiu et al. (29) also proposed from theoretical MD simulations that the hydroxyl group of cholesterol adds a contribution to the dipole potential and in addition proposed an effect arising from increased water penetration into the bilayer.
Considering the disagreement in the theoretical studies, further experimental data would be desirable to test some of the theoretical hypotheses. Fortunately, a spectroscopic method has recently been developed that allows a relatively simple quantification of the magnitude of the dipole potential of lipid vesicles (30,31). The method involves the use of the voltage-sensitive fluorescent styrylpyridinium dye, di-8-ANEPPS. Since the dye binds strongly at the membrane-water interface and its fluorescence excitation spectrum is very sensitive to its local electrical field, it can be used as an effective probe of membrane dipole potential. Using this technique we have monitored the effect of increasing concentrations of cholesterol and four of its derivatives (6-ketocholestanol, 4-cholesten-3-one, coprostanol, and 5-cholesten-3β-ol-7-one; see Fig. 1 for the structures) on the dipole potential of DMPC lipid vesicles. A further advantage of this technique is that it allows measurements on lipid vesicles in solution in contrast to most other methods of dipole potential determination, which are carried out on planar lipid monolayers or bilayers. The technique is, therefore, well suited to the study of natural membrane systems as well as synthetic membranes. A further aim of this work was, therefore, to establish that cholesterol does in fact cause an increase in the dipole potential in vesicles reconstituted from natural membrane lipids. For this purpose we used lipids extracted from the kidney and brain of eight different vertebrate species.
FIGURE 1.
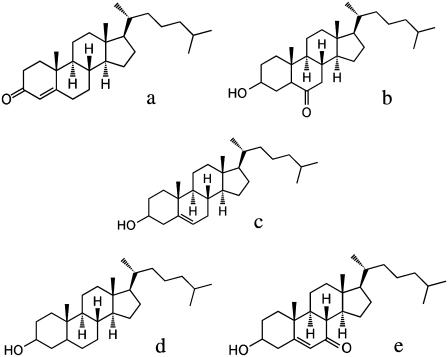
Chemical structures of cholesterol and its derivatives. (a) 4-Cholesten-3-one, (b) 6-ketocholestanol, (c) cholesterol, (d) coprostanol, and (e) 5-cholesten-3β-ol-7-one.
MATERIALS AND METHODS
Natural lipid vesicle preparation and fluorescence measurements
4-(2-(6-(Dioctylamino)-2-naphthalenyl)ethenyl)-1-(3-sulfopropyl)-pyridinium inner salt (di-8-ANEPPS) was obtained from Molecular Probes (Eugene, OR) and was used without further purification.
Animals examined in this study were adults of either sex. They were obtained, maintained, and killed by procedures described in detail elsewhere (17). All animal procedures were performed in accordance with the National Health and Medical Research Council Guidelines for Animal Research in Australia and were approved by the Animal Experimentation Ethics committee of the University of Wollongong. Kidneys and brains were removed after the death of each animal. Samples of each tissue were kept at −80°C. Microsomal membrane fragments were prepared according to the procedure of Jørgensen (32). Tissue homogenates (10% in 250 mM sucrose, 30 mM histidine; pH 7.4) were centrifuged at 6,000 × g for 15 min with the pellet resuspended and centrifuged for a further 15 min at 6,000 × g. The supernatants from both spins were combined and centrifuged at 48,000 × g for 35 min. The resultant pellets, designated microsomal membranes, were resuspended in 250 mM sucrose, 30 mM histidine (pH 7.2).
Lipids were extracted from microsomal membrane fractions by a standard method (33) using chloroform/methanol (2:1 vol/vol) containing butylated hydroxytoluene (0.01% wt/vol) as an antioxidant. In this study we have termed these extracts “total membrane lipids” because they include both phospholipids and other membrane lipids (primarily cholesterol). Phospholipids were separated by solid-phase extraction on Phenomenex SPE silica cartridges (Pennant Hills, New South Wales, Australia). The phospholipid concentration of the total membrane lipids and the phospholipids was determined using a phosphorus assay as described by Mills et al. (34). All solvents used in the lipid extractions were of ultrapure grade and were from Merck (Kilsyth, Victoria, Australia). Analytical grade butylated hydroxytoluene was from Sigma-Aldrich (Castle Hill, New South Wales, Australia).
To prepare vesicles from the natural lipids, total membrane lipid and phospholipid extracts (∼2 mM) from microsomal preparations were dried under nitrogen and resuspended in a buffer containing 30 mM Tris, 1 mM EDTA, and 150 mM NaCl in deionized water (pH 7.2). The resuspension process involved 20–30 min of sonication under nitrogen to prevent any oxidation. Total lipid and phospholipid suspensions were then given 11 passes through an Avanti (Alabaster, AL) Mini-Extruder, equipped with a 100-nm pore size polycarbonate membrane to produce unilamellar lipid vesicles.
To 1 ml of each lipid suspension 5 μl of a 1.0 mM ethanolic solution of di-8-ANEPPS were added and the suspensions were left overnight at 30°C to allow for dye disaggregation and incorporation into the membrane. Steady-state fluorescence excitation spectra were then recorded at 30°C at an emission wavelength of 670 nm (+RG645 glass cutoff filter) using a Shimadzu (Rydalmere, Australia) RF-5301PC spectrofluorophotometer. After excitation correction (using a rhodamine B quantum counter), the fluorescence ratio, R (defined as the fluorescence intensity at an excitation wavelength of 420 nm divided by that at 520 nm), was calculated. The wavelengths were chosen based on a previous study (35) to avoid any effects of membrane fluidity on the measured fluorescence ratios. The R values were then converted into dipole potential, ψd, values in millivolts according to the linear relationship ψd = (R − d)/m, where m = 4.3 (± 1.2) × 10−3 mV−1 and d = −0.3 (± 0.4). This equation is based on a calibration of the fluorescence ratios against electrical measurements of the dipole potential for a series of pure lipids and has been described in detail elsewhere (17). In the case of experiments on methyl-β-cyclodextrin-treated vesicles, before the addition of di-8-ANEPPS, microsomes were first equilibrated for 30 min with 30 mM methyl-β-cyclodextrin (Sigma-Aldrich), after which the lipids were extracted and vesicles were prepared as described above. Methyl-β-cyclodextrin forms an inclusion compound with cholesterol and effectively withdraws cholesterol out of the membrane into the aqueous phase (19,36–38). The cholesterol content of the total lipid vesicles was assayed before and after methyl-β-cyclodextrin treatment using the Sigma Procedure No. 352 enzymatic assay kit.
Synthetic lipid vesicle preparation and fluorescence measurements
DMPC and dioleoylphosphatidylcholine (DOPC) were obtained from Avanti Polar Lipids. Egg PC was obtained from Sigma-Aldrich. Unilamellar vesicles were prepared by extrusion above the main phase transition temperature (i.e., at 30°C for DMPC and at room temperature for DOPC and egg PC) through a 100-nm pore size Nucleopore polycarbonate membrane using an Avanti Mini-Extruder. Before extrusion, the PC and any cholesterol (or derivative) required were dissolved in chloroform, dried to form a lipid film, and resuspended in a buffer containing 30 mM Tris, 1 mM EDTA, and 150 mM NaCl. The pH of the buffer was adjusted to 7.2 with HCl. The resuspension process involved 20–30 min of sonication. Cholesterol and all of its derivatives studied were from Sigma-Aldrich. The PC concentration for all vesicle preparations was ∼3.6 mM.
The addition of di-8-ANEPPS to the synthetic lipid vesicles and the fluorescence measurements to determine the dipole potential were carried out following the same procedure as for the natural vesicles (see above).
Computational methods
The structures of cholesterol and each derivative were optimized using the B3LYP hybrid density functional using the 6-31G basis set. The dipole moments were then calculated in a vacuum using the same functional with added polarization and diffuse functions with the 6-31+G* basis set. The angle between the dipole moments and the axis normal to the membrane surface (defined to be along the C−O bond linking the sterol ring system to the hydroxyl group or carbonyl oxygen in the case of 4-cholesten-3-one) was calculated from the dot product of the dipole moment vector and a vector along the C−O bond according to θ = arccos [(μx vx + μy vy + μz vz)/(|μ | |v|)], where μx, μy, and μz are the coordinates of the dipole moment vector, vx, vy, and vy are those of the vector along the C−O bond, |μ | is the total length of the dipole moment vector, and |v| is the total length of the C−O bond. The component of each dipole moment along the bilayer normal, μ⊥, was then simply calculated from μ⊥ = |μ | cosθ.
RESULTS
Cholesterol extraction from natural lipid vesicles
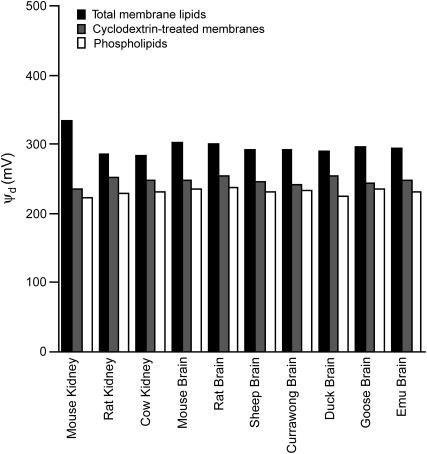
Here we have explored the effect of cholesterol depletion on the dipole potential of natural membrane lipids isolated from brain and kidney from eight mammalian and avian species. Treatment of vesicles from total membrane lipids with methyl-β-cyclodextrin (30 mM) removed on average 80% (range 68–90%) of the membrane cholesterol (see Table 1). Using the dipole potential probe di-8-ANEPPS (30,31), we found that this reduction in cholesterol content was associated with an average drop in the dipole potential of 50 mV (see Fig. 2). As can be seen from Fig. 2, the dipole potential of the total membrane lipids (i.e., phospholipids plus cholesterol) was on average 297 mV, the dipole potential of the phospholipids only (i.e., no cholesterol) averaged as 232 mV, whereas the dipole potential of the cyclodextrin-treated total membrane lipids was on average 247 mV. The cholesterol contribution to the dipole potential of the total membrane lipids thus amounts to 65 mV (297 − 232). By examining the change in dipole potential of the cyclodextrin-treated membranes relative to the dipole potential of both the total membrane lipids and the phospholipids, one finds that an 80% reduction in the cholesterol content of the membrane results in a 77% reduction in the cholesterol contribution to the total dipole potential, i.e., 50 mV drop/65 mV cholesterol contribution. There is, therefore, a very good agreement between the amount of cholesterol removed by cyclodextrin treatment and the amount by which its contribution to the dipole potential is reduced. Hence, one can confidently state that in vesicles composed of natural membrane lipids, cholesterol is responsible for a significant increase in the dipole potential, as has previously been observed in synthetic model membrane vesicles (19,30) and intact cells (19).
TABLE 1.
Reduction in microsomal membrane cholesterol levels resulting in methyl-β-cyclodextrin treatment
| Cholesterol (μg.mg protein−1) |
Phospholipid (μg.mg protein−1) |
Cholesterol/phospholipid (mole/mole)* |
||||
|---|---|---|---|---|---|---|
| Total membrane lipids | Cyclodextrin-treated membranes | Total membrane lipids | Cyclodextrin-treated membranes | Total membrane lipids | Cyclodextrin-treated membranes | |
| Kidney | ||||||
| Mouse | 49 | 9 | 325 | 408 | 0.30 | 0.04 |
| Rat | 56 | 12 | 396 | 485 | 0.29 | 0.05 |
| Cow | 60 | 14 | 282 | 651 | 0.43 | 0.04 |
| Brain | ||||||
| Mouse | 101 | 29 | 421 | 619 | 0.48 | 0.10 |
| Rat | 124 | 22 | 668 | 1027 | 0.37 | 0.04 |
| Sheep | 124 | 30 | 532 | 875 | 0.47 | 0.07 |
| Currawong | 105 | 30 | 570 | 698 | 0.37 | 0.09 |
| Duck | 94 | 34 | 525 | 615 | 0.36 | 0.11 |
| Goose | 86 | 32 | 528 | 695 | 0.33 | 0.09 |
| Emu | 78 | 38 | 455 | 743 | 0.35 | 0.10 |
The moles of phospholipids were estimated assuming an average molecular mass of 780 g mol−1.
FIGURE 2.
Reduction in membrane dipole potential, ψd, resulting from methyl-β-cyclodextrin treatment. Membrane dipole potential values (in millivolts) were determined in lipid vesicles isolated from microsomal membrane preparations by measuring the di-8-ANEPPS fluorescence ratio, R (see definition under Materials and Methods), which is related to ψd by the equation ψd = (R + 0.3)/(4.3 × 10−3) (17). Shown above are the dipole potential values for total membrane lipids (i.e., phospholipids plus cholesterol), phospholipids only, and membranes treated for 30 min with 30 mM methyl-β-cyclodextrin, where ∼80% of cholesterol was removed.
Interaction of cholesterol and its derivatives with phosphatidylcholine vesicles
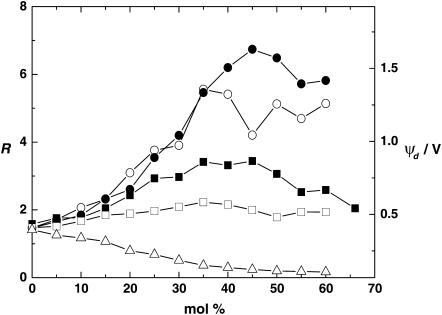
Using the di-8-ANEPPS fluorescence ratiometric method, the dipole potential of DMPC lipid vesicles containing increasing concentrations of either cholesterol or one of its derivatives (6-ketocholestanol, 4-cholesten-3-one, coprostanol, and 5-cholesten-3β-ol-7-one) was measured. The results obtained are shown in Fig. 3. It can easily be seen that there is a wide variation in the effects of the cholesterol derivatives on ψd, despite their quite subtle structural differences. Relative to the dipole potential of pure DMPC vesicles, cholesterol, 6-ketocholestanol, 4-cholesten-3-one, and coprostanol all cause an increase in ψd to varying degrees, whereas cholesten-3β-ol-7-one causes a decrease.
FIGURE 3.
Effect of cholesterol derivative concentration on the fluorescence ratio, R, and the dipole potential, ψd. [di-8-ANEPPS] = 5 μM, [DMPC] = 3.6 mM, 30°C. (•) 6-Ketocholestanol, (○) 4-cholesten-3-one, (▪) cholesterol, (□) coprostanol, and (Δ) cholesten-3β-ol-7-one. Each individual point corresponds to a separate vesicle preparation at the given DMPC and cholesterol compositions.
Each point plotted on Fig. 3 refers to a separate vesicle preparation at the given composition and represents an average of five individual fluorescence measurements. Minor deviations in the course of each curve are most likely due to experimental variability in the exact composition of given preparation.
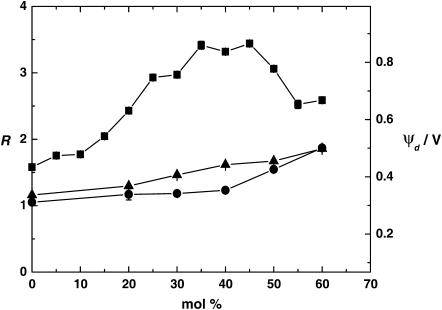
To investigate the effect of PC chain saturation on the cholesterol-induced dipole potential changes, experiments were also performed using DOPC (18:1) and egg PC. DOPC has fatty acid chain lengths of 18 carbons, with a single double bond in each chain, whereas the chains of DMPC (14:0) are 14 carbons in length with no double bonds. Egg PC is a natural lipid extract whose major components are dipalmitoylphosphatidylcholine (16:0) 32%, distearoylphosphatidylcholine (18:0) 16%, DOPC (18:1) 30%, dilinoleoylphosphatidylcholine (18:2) 17%, and diarachidonylphosphatidylcholine (20:4) 3% (39). The results obtained are shown in Fig. 4. The data for DMPC has been included in the figure for comparison. It can be clearly seen that cholesterol causes an increase in the dipole potential of both DOPC and egg PC. However, the magnitude of the change is significantly smaller than that observed in DMPC.
FIGURE 4.
Effect of cholesterol concentration on the fluorescence ratio, R, and the dipole potential, ψd, of DMPC (▪), egg PC (•), and DOPC (▴) vesicles. [di-8-ANEPPS] = 5 μM, [PC] = 3.6 mM. For DMPC, the measurements were performed at 30°C. For DOPC and egg PC, the measurements were performed at room temperature.
Dipole moment calculations
To explain the varying effects of each cholesterol derivative on ψd we calculated quantum mechanically at the B3LYP/6-31G* level of theory the dipole moment, μ, of each derivative in a vacuum. Based on the known orientation of cholesterol in PC bilayers from x-ray diffraction studies (40,41), we then calculated the component of the total μ of each derivative along an axis normal to the membrane surface, μ⊥ (see Table 2). Considering their very similar structures, we assumed that the orientation in the membrane of each derivative is identical, and the membrane normal axis was taken to lie along the C−O bond linking the sterol ring system to the hydroxyl group (or the carbonyl oxygen, in the case of 4-cholesten-3-one). The assumption of identical orientations of each derivative would seem to be justified, since the primary driving force for insertion of cholesterol and its derivatives into the membrane would be expected to be the hydrophobic interaction of its hydrocarbon chain with the hydrocarbon interior of the membrane. The hydrophilic 3-hydroxy group of cholesterol, on the other hand, is known to be situated close to the carbonyl groups of the ester linkage between the PC headgroup and the hydrocarbon chains (42). There is evidence based on the kinetics of cholesterol dissociation from diester- and diether-phosphatidylcholine vesicles that the 3-hydroxy group of cholesterol hydrogen bonds with the carbonyl groups of diester-phosphatidylcholine (43). If such hydrogen bonding occurs, it is likely that it would occur in all cholesterol derivatives containing the 3-hydroxy residue and that this would be a further factor contributing to very similar membrane orientations of the cholesterol derivatives studied here. Specific hydrogen bonding between cholesterol and the oxygens on the phosphate group of PC, on the other hand, does not appear to be supported by 31P-NMR studies (44).
TABLE 2.
Dipole moments of the cholesterol derivatives
| Cholesterol derivative | μ/D* | θ/deg† | μ⊥/D‡ | R at 40 mol %§ | Δψd/mV¶ |
|---|---|---|---|---|---|
| 4-Cholestene-3-one | 5.6 | 6.0 | 5.5 | 5.41 (± 0.08) | 913 (± 409) |
| 6-Ketocholestanol | 4.0 | 30.4 | 3.5 | 6.20 (± 0.02) | 1095 (± 455) |
| cholesterol | 1.9 | 35.5 | 1.5 | 3.32 (± 0.04) | 426 (± 292) |
| coprostanol | 2.0 | 41.3 | 1.5 | 2.16 (± 0.01) | 157 (± 236) |
| 5-Cholesten-3β-ol-7-one | 2.6 | 117.3 | −1.2 | 0.296 (± 0.005) | −275 (± 178) |
μ is the total dipole moment (in Debye) in a vacuum.
θ is the angle between the dipole moment vector and an axis perpendicular to the surface of the bilayer.
μ⊥ is the component of the dipole moment perpendicular to the surface of the bilayer.
R is the fluorescence ratio at 40 mol % of each derivative. The values of the errors quoted represent the standard deviations of five measurements on a single vesicle preparation.
Δψd is the change in dipole potential on going from pure DMPC to 40 mol % of each cholesterol derivative.
To ensure confidence in the theoretically calculated dipole moments, we also compared our values with experimental literature data. Experimentally the dipole moment of cholesterol has been determined to be 2.01 D (45), which is in good agreement with our total dipole moment value of 1.9 D. The dipole moment of coprostanol has been reported to be 1.83 D (45). Here we calculated a value of 2.0 D. Therefore, the theoretically calculated dipole moments can be confidently considered to be good estimates of the actual values.
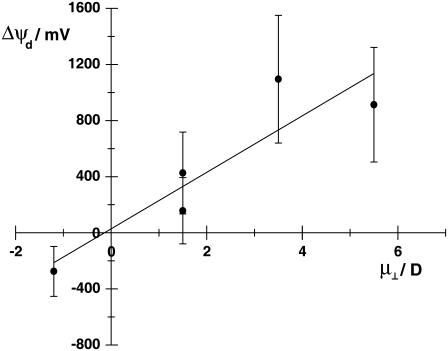
Comparison of the experimentally determined effect of each cholesterol derivative on the fluorescence ratio, R, or on the change in dipole potential, Δψd, with the component of the dipole moment perpendicular to the membrane surface, μ⊥ (see Table 2), shows that the effect of each cholesterol derivative on the magnitude and sign of ψd correlates well with the magnitude and direction of the molecule's μ⊥. 6-ketocholestanol, 4-cholesten-3-one, cholesterol, and coprostanol, which all have the positive end of their dipole pointing toward the membrane interior, cause an increase in ψd. 5-Cholesten-3β-ol-7-one, in contrast, has the positive end of its dipole pointing toward the aqueous phase and causes a decrease in ψd. The correlation between μ⊥ and Δψd at 40 mol % of each cholesterol derivative is shown graphically in Fig. 5. Fitting a straight line to the data yields a slope of 191 (± 40) mV D−1 and a y-intercept of 35 (± 124) mV. It should be noted that the large errors in the absolute values of ψd are in fact due to the variation in literature electrical data used to calibrate the fluorescent probe (cf. Starke-Peterkovic (17)), not to the fluorescence ratio method itself, which is very precise as evidenced by the small errors in R shown in Table 2, i.e., at most ±1.4%.
FIGURE 5.
Correlation between the change in dipole potential, Δψd (in millivolts), of 40 mol % of each cholesterol derivative relative to the dipole potential of pure DMPC lipid vesicles and the dipole moment of each cholesterol derivative perpendicular to the surface of the membrane, μ⊥ (in Debye). The solid line represents a least squares fit of the data to a straight line. It is described by the equation Δψd = 201 (± 54) × μ⊥ + 28 (± 170).
DISCUSSION
The correlation observed between the experimentally measured effect of each cholesterol derivative on the membrane dipole potential and the theoretical value of their dipole moment perpendicular to the membrane surface (see Fig. 5) indicates that the cholesterol derivatives modify ψd either directly via the electric field of their intrinsic dipole moments or indirectly via an effect on some other membrane property, e.g., lipid packing density or polarization of interfacial water. If the effect is indirect, the observed correlation implies that whatever the effect is, it is also determined by the strength of the derivative's dipole moment.
To determine whether the intrinsic dipole moments of the cholesterol derivatives could alone account for the experimentally observed ψd changes, we used our theoretically determined μ⊥ values and calculated the theoretical change in ψd expected based on the Helmholtz equation for a parallel-plate capacitor,
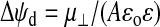 |
(1) |
where A is the average total surface area of membrane (including surrounding DMPC molecules) containing one cholesterol derivative molecule, ɛo is the permittivity of free space, and ɛ is the headgroup region's effective dielectric constant. This equation predicts a linear relationship between the change in dipole potential, Δψd, and the dipole packing density, μ⊥/A, which should pass through the origin. The prediction of the equation is, thus, in agreement with the experimentally observed behavior (see Fig. 5). For our calculation we assumed a value of 10 for ɛ (46) and we carried out the calculation at 40 mol % using a value for A of 1.07 nm2. The latter value was estimated from the molecular areas of 0.539 nm2 for DMPC and 0.266 nm2 for cholesterol at 40 mol % and 30°C (47). As a rough approximation, the same values were assumed to apply to all of the cholesterol derivatives studied. The results of the calculations yielded Δψd values of 124 mV, 195 mV, 53 mV, 53 mV, and −43 mV for the molecules 6-ketocholestanol, 4-cholesten-3-one, cholesterol, coprostanol, and 5-cholesten-3β-ol-7-one. Experimentally (see Fig. 3) the values of Δψd are, however, much larger, i.e., 1095 mV, 913 mV, 426 mV, 157 mV, and −275 mV for the same molecules. Thus, it appears that the intrinsic dipole moments of the cholesterol derivatives only account directly via the electric field they produce for ∼10–30% of the total change in dipole potential.
The same conclusion is reached if one considers the slope of the data shown in Fig. 5. According to the Helmholtz equation (Eq. 1), the slope of the curve should equal 1/Aɛoɛ. The calculated slope was 201 (± 54) mV D−1, which on conversion to SI units corresponds to 6.0 (± 1.6) × 1028 VC−1m−1. Again assuming an effective dielectric constant of 10, the area per cholesterol derivative which would be necessary to totally account for the dipole potential changes can be estimated from this value to be 0.188 (± 0.050) nm2. In fact, this is only 18% of the estimated value of the area per cholesterol molecule in a DMPC membrane at 40 mol % of cholesterol, i.e., 1.07 nm2 (see above). Therefore, this calculation also shows that the dipole moments of the cholesterol derivatives themselves are directly responsible for only ∼20% of the total effect on the membrane dipole potential. Presumably the rest of the dipole potential change must arise from a further indirect effect of the cholesterol derivatives' dipole moments.
Based on MD simulations Tu et al. (48) suggested an interesting mechanism of cholesterol modification of the dipole potential, whereby the lipid headgroups rotate toward the bilayer to fill spaces left by the cholesterol molecules, which sit more deeply in the membrane. They suggest that this mechanism would lead to a reduced compensation of the contribution of oriented water to the dipole potential by the dipoles of the headgroups. Hence a net increase in dipole potential would be expected. This study shows experimentally that, if such a mechanism does occur, it can only occur as a consequence of electrostatic attraction from the cholesterol molecule. All of the cholesterol derivatives studied here have similar sizes, but they have varying effects on the dipole potential. Thus, any reorientation of the headgroup which might serve merely to optimize lipid-cholesterol packing and maximize dispersion interactions can be excluded as a general mechanism for the dipole potential changes.
A more likely mechanism which could contribute to the greater than expected effect of cholesterol and its derivatives on the dipole potential is via lipid packing density. The change in dipole potential calculated above from Eq. 1 was the change in ψd arising from the dipole moments of the cholesterol derivatives themselves. In a pure DMPC membrane, however, the dipole potential arises from dipoles associated with the lipid headgroups, the carbonyl bonds of the ester linkages, and oriented water molecules in the headgroup region (6–8). Therefore, any cholesterol-induced change in the density of packing of the lipid molecules and their associated water molecules would be expected to produce a further change in ψd. It has in fact long been known from lipid monolayer studies that cholesterol has a condensing effect on PC membranes (44,49). From x-ray scattering data (50) it was found that the partial molecular area of PC in egg PC/cholesterol mixtures containing 25% water decreased with increasing cholesterol mole fraction from ∼61 Å2 in the absence of cholesterol to ∼49 Å2 in the presence of 40 mol % cholesterol. Similar values are obtained from NMR studies (51) and monolayer studies (49,50) carried out at the surface pressure expected for a biological membrane of 30 mN m−1 (52). For DMPC at 30°C, Almeida et al. (47) have reported a drop in area per DMPC molecule from 58.5 Å2 in the absence of cholesterol to 53.8 Å2 in the presence of 40 mol % cholesterol, i.e., an 8.7% increase in packing density (1/A) of the DMPC molecules on increasing the cholesterol content from 0 to 40 mol %. Hence, according to Eq. 1, one would expect an 8.7% increase in ψd. The dipole potential of pure DMPC can be calculated from the R value via the equation ψd = (R + 0.3)/(4.3 × 10−3) (17) to be 411 (± 148) mV. An 8.7% increase in ψd, thus, corresponds to an absolute increase of 36 mV. This is in addition to the 53 mV increase in ψd expected due to the dipole moment of cholesterol itself. Combining the two effects yields a total expected increase in ψd at 40 mol % cholesterol of 89 mV. This is, however, still much smaller than the experimentally determined change of 426 mV.
If lipid condensation is contributing to the change in ψd observed on addition of cholesterol, it would imply that 5-cholesten-3β-ol-7-one should produce an expansion of the lipid packing. Whereas all the other cholesterol derivatives studied produced an increase in ψd, 5-cholesten-3β-ol-7-one produced a decrease in ψd (see Fig. 3). This will certainly require further experimental investigation. The correlation seen between Δψd and μ⊥ (see Fig. 5) would, furthermore, seem to imply that a major driving force for the cholesterol condensation effect is dipole-dipole interaction between the cholesterol derivatives and the PC molecules, whereas in the past it has generally been accepted to be due to van der Waals interactions between the cholesterol ring structure and the phospholipids hydrocarbon chains (51).
The calculations which we have performed so far indicate that the combined contributions to ψd from the cholesterol derivatives' intrinsic dipole moments and from changes in the packing of the PC headgroups do not totally explain the large changes in ψd observed. There must be a further additional mechanism. Another likely effect is a cholesterol-induced change in dielectric constant, ɛ, of the lipid headgroup region. From Eq. 1, it can be seen that if ɛ decreases, Δψd could be significantly increased. From combined x-ray diffraction and electrical membrane capacitance measurements, Simon and McIntosh (53) showed that the incorporation of cholesterol into a bilayer membrane composed of bacterial phosphatidylethanolamine significantly decreases water penetration into the bilayer and correspondingly increases the thickness of the region of low dielectric constant (i.e., 2.2). In the absence of cholesterol they found that water penetrates to near the deeper carbonyl group of the ester linkage to the hydrocarbon chains. In a 1:1 cholesterol/phosphatidylethanolamine mixture, however, they found that water penetrated only to a position near the glycerol backbone. Therefore, one would expect cholesterol to cause a significant drop in the dielectric constant in the region of the ester carbonyl groups of the phospholipids. If the same effect occurs in PC bilayers, which would seem to be very likely, one could easily envisage a maximal drop in ɛ from ∼10 down to a value as low as 2.2 in the membrane region where the dipole potential is located. According to Eq. 1, this could produce a 355% increase in both the PC dipole contribution as well as the cholesterol dipole contribution to ψd. The exact magnitude of this effect is difficult to quantify, since the exact magnitudes of the dielectric constants are difficult to estimate and the exact location of the electrical potential drop due to the dipole potential is not precisely known. Nevertheless, the simple calculation shown here demonstrates that the effect is more than enough to account for the rest of the experimentally observed dipole potential change.
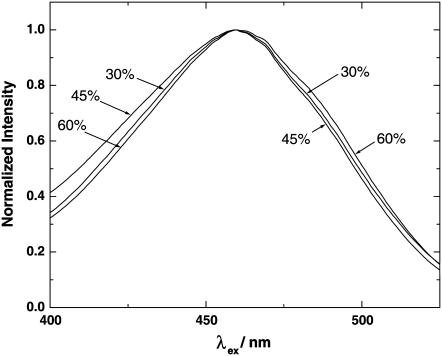
Interestingly, in the case of DMPC the results show (see Fig. 3) that for those derivatives which cause an increase in ψd (including cholesterol), a maximum in ψd is reached in the region 35–45 mol %. This change in direction of ψd is observable as a change in the direction of the shift of the fluorescence excitation spectrum of di-8-ANEPPS (see Fig. 6). On going from 30 to 45 mol % cholesterol, one sees a blue shift of the spectrum, but on going from 45 to 60 mol % the spectrum shifts back toward the red. A possible explanation for this change in direction is that a phase change of the membrane could occur at this point. Vist and Davis (54) presented evidence for the formation of a liquid-ordered state (characteristic of “lipid rafts”) when the cholesterol content of a DPPC/cholesterol mixture exceeds 25%. Simulations of Smondreyev and Berkowitz (55) indicate that such a phase change is likely to be associated with changes in the tilts of the cholesterol and lipid molecules, which would affect ψd. Lateral separation into cholesterol-rich and PC-rich regions is another possibility, which could occur at high cholesterol content and could affect ψd. However, we would like to propose a simpler explanation which does not involve any phase transition. Maxima or minima in the physical properties of two-component solvent mixtures are often observed and are generally attributed to changes in the strength of intermolecular forces as the solvent composition varies. In the case of boiling points, the maxima or minima are termed azeotropes. A simple example is the boiling point of the acetone/hexane system. An azeotropic mixture of 65% acetone and 35% hexane has a boiling point significantly below that of both pure acetone and pure hexane, i.e., a minimum in the boiling point is observed at this point. This can easily be explained by the fact that the intermolecular forces between an acetone molecule and a hexane molecule are weaker than those between two acetone molecules or between two hexane molecules. Considering a PC/cholesterol membrane as a two-component two-dimensional solvent mixture, the maximum in the dipole potential observed in Fig. 3 can be explained in a similar fashion. If the dipole potentials of a pure DMPC membrane and a hypothetical pure cholesterol membrane were relatively similar, a maximum in the dipole potential would be expected if the intermolecular forces between two cholesterol molecules and between two PC molecules were both weaker than those between a cholesterol molecule and a PC molecule. At low mol % values of cholesterol, stronger intermolecular forces between cholesterol and PC would cause a condensation of the membrane (as experimentally observed in both monolayer and NMR studies (44,49–51)) and an increase in ψd would be observed due to the higher packing density. At a particular cholesterol composition a maximum would then be reached, because at high mole percentages of cholesterol the weaker intermolecular forces between two cholesterol molecules would take over and cause an expansion of the bilayer. Parker et al. (56) have suggested that the intermolecular forces between two cholesterol molecules in a membrane are in fact weak, because their small hydroxyl group cannot adequately shield the hydrophobic portion of the molecule from water. In contrast, this is done much more effectively by a PC molecule. This could then easily lead to cholesterol spacing itself out in the membrane and to the formation of regular hexagonal superlattice structures, as proposed by a number of authors (56–58). In addition, as mentioned earlier, some authors have suggested a more specific interaction between PC and cholesterol via hydrogen bonding (43,59).
FIGURE 6.
Normalized corrected fluorescence excitation spectra of DMPC/cholesterol vesicles labeled with di-8-ANEPPS at 30, 45, and 60 mol % cholesterol. [di-8-ANEPPS] = 5 μM, [DMPC] = 3.6 mM, 30°C. The fluorescence emission was measured at 670 nm (+RG645 glass cutoff filter).
In the case of the measurements performed on DOPC and egg PC vesicles, in contrast, only an increase in ψd was observed (see Fig. 4), i.e., no change in direction of the cholesterol effect on ψd was found. The magnitudes of the observed changes were also smaller than in the case of DMPC. This is likely to be due to the presence of unsaturated hydrocarbon chains in DOPC and egg PC. An important effect of chain unsaturation is to decrease the order of the chains within the membrane, because the introduction of cis double bonds causes a kink in the chain and hence decreases the effectiveness of chain packing. This is the reason both pure DOPC and egg PC have a lower ψd than DMPC (31). Because of the decreased packing of DOPC, one would expect the intermolecular forces between any cholesterol which inserts itself into the membrane and adjacent DOPC molecules to be weaker than in the case of DMPC. This could explain the smaller effect of cholesterol on ψd in the case of DOPC and egg PC. This interpretation is consistent with the electron spin resonance study of Shin et al. (60), which indicated a preference for cholesterol to interact with saturated PCs over unsaturated ones, and the NMR study of Huster et al. (61), showing that the saturated chains of mixed chain lipids orient preferentially toward cholesterol. It is also supported by the kinetic results of Lund-Katz et al. (62), showing that the rate constant for cholesterol desorption is much greater for unsaturated PCs than saturated ones. They proposed on the basis of their results that a stronger van der Waals attraction between cholesterol and saturated PCs was responsible for the kinetic difference.
In the mol % region of 25–50% relevant for lipid rafts it can be seen that increases in ψd of 500–750 mV over pure PC membranes can be expected. This is likely to have major effects on the function of raft-localized proteins. It is worth noting that biphasic effects of cholesterol on the kinetics of a membrane-bound enzyme, the Na+,K+-ATPase, have been observed (63,64). At low concentrations cholesterol stimulates the kinetics of the enzyme, whereas at high concentrations it is inhibitory. Extraction of cholesterol via methyl-β-cyclodextrin has also recently been shown to decrease Na+,K+-ATPase activity in vivo in renal epithelial cells (65). Both Yeagle et al. (63) and Sotomayor et al. (64) found a maximum in activity at the cholesterol concentration corresponding to the physiological level in the tissue they were using. Cornelius (66) has suggested that cholesterol may change the activity of the Na+,K+-ATPase by its membrane condensing effect, which also leads to an increase in bilayer thickness due to ordering of the hydrocarbon chains. If the membrane thickness changes, this can then lead to a change in the “hydrophobic matching” between the hydrophobic transmembrane portions of the enzyme (which have a constant thickness) and the hydrophobic interior of the membrane. Based on fluorescent measurements using a probe which is sensitive to hydration at the membrane-water interface, Sotomayor et al. (64) have suggested another mechanism, whereby cholesterol induces a change in hydration at the protein-lipid interface. They have suggested that hydration/dehydration reactions are likely to contribute more to the energetics of conformational changes of membrane proteins than generally thought. The maximum in the dipole potential observed here also occurs at a cholesterol concentration which corresponds closely to the physiological concentration in kidney membranes (63). The suggestion of Sotomayor et al. (64) that cholesterol induces changes in hydration is entirely consistent with our results showing changes in dipole potential since, as described above, changes in water penetration into the membrane would be expected to induce significant changes in dipole potential. Since both of these effects are interwoven, it is very difficult to say whether the Na+,K+-ATPase activity is being influenced by the change in interface hydration or by the change in dipole potential or by both. Changes in the rate constants and equilibrium constants of the Na+,K+-ATPase by lyotropic anions, which decrease the membrane dipole potential, have, however, been observed by Ganea et al. (67). A dependence of the steady-state activity of the Na+,K+-ATPase from a variety of animals on the dipole potential of their natural lipid extracts was also indicated by the results of Starke-Peterkovic et al. (17). The dependence observed there seemed to be best explained by an exponential curve. Such behavior could easily be explained from a theoretical basis, because a change in dipole potential could change the activation energy of a rate-determining charge-translocating step of the enzyme cycle. Because the activation energy appears in the exponential term of the Arrhenius equation, such an effect could indeed lead to an exponential dependence of the steady-state activity on the dipole potential. Steady-state enzyme activity, however, also depends on the degree of saturation of the ion-binding sites, in particular the cytoplasmic Na+ and K+ sites (68). If these ion-binding steps involve charge movement within the membrane, then an exponential dependence of steady-state activity on the dipole potential would also be theoretically expected via an exponential Boltzmann relation for the ion-binding constants.
Finally it should be noted that 5-cholesten-3β-ol-7-one (often referred to as 7-ketocholesterol) was the only cholesterol derivative studied here which caused a decrease in ψd in comparison to pure DMPC. This cholesterol derivative is of particular medical interest, because it was recently shown to accumulate in atherosclerotic plaques and to lead vascular cells to apoptosis (69). It is feasible that the unusual decrease in ψd induced by 5-cholesten-3β-ol-7-one could influence the function of proteins involved in the signaling pathways leading to cell death.
Acknowledgments
We thank Dr. George Bacskay for valuable discussions and suggestions and Dr. Christian Lüpfert for preliminary investigations.
R. J. Clarke acknowledges with gratitude financial support from the Australian Research Council (Discovery Grant DP-0208282) and from the Australian Research Council/National Health and Medical Research Council-funded Research Network “Fluorescence Applications in Biotechnology and Life Sciences” (RN0460002). M. F. Vitha acknowledges the Donors of the American Chemical Society Petroleum Research Fund for partial support of this research.
Thomas Starke-Peterkovic's present address is Groupe de Biophysique, Laboratoire de Chimie-Physique, Bât. 349, Universite d'Orsay, 91400 Orsay, France.
Nigel Turner's present address is Molecular Metabolism Group, Garvan Institute of Medical Research, 384 Victoria St., Darlinghurst, NSW 2010, Australia.
References
- 1.Pike, L. J. 2004. Lipid rafts: heterogeneity on the high seas. Biochem. J. 378:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szabo, G. 1974. Dual mechanism for the action of cholesterol on membrane permeability. Nature. 252:47–49. [DOI] [PubMed] [Google Scholar]
- 3.Krull, U. J., M. Thompson, and H. E. Wong. 1986. Chemical modification of the bilayer lipid membrane biosensor dipolar potential. Bioelectrochem. Bioenerg. 15:371–382. [Google Scholar]
- 4.Krull, U. J. 1987. Bilayer lipid membrane ion permeability. An empirical model based on molecular packing/fluidity and membrane dipolar potentials. J. Electrochem. Soc. 134:1910–1914. [Google Scholar]
- 5.McIntosh, T. J., A. D. Magid, and S. A. Simon. 1989. Cholesterol modifies the short-range repulsive interactions between phosphatidylcholine membranes. Biochemistry. 28:17–25. [DOI] [PubMed] [Google Scholar]
- 6.Gawrisch, K., D. Ruston, J. Zimmerberg, V. A. Parsegian, R. P. Rand, and N. Fuller. 1992. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys. J. 61:1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brockman, H. 1994. Dipole potential of lipid membranes. Chem. Phys. Lipids. 73:57–79. [DOI] [PubMed] [Google Scholar]
- 8.Peterson, U., D. A. Mannock, R. N. A. H. Lewis, P. Pohl, R. N. McElhaney, and E. E. Pohl. 2002. Origin of membrane dipole potential: contribution of the phospholipid fatty acid chains. Chem. Phys. Lipids. 117:19–27. [DOI] [PubMed] [Google Scholar]
- 9.Schamberger, J., and R. J. Clarke. 2002. Hydrophobic ion hydration and the magnitude of the dipole potential. Biophys. J. 82:3081–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Revell Phillips, L., C. D. Cole, R. J. Hendershot, M. Cotton, T. A. Cross, and D. D. Busath. 1999. Noncontact dipole effects on channel permeation. III. Anomalous proton conductance effects in gramicidin. Biophys. J. 77:2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffin, R. L., M. P. Garrett, K. B. Flake, J. D. Durrant, and D. D. Busath. 2003. Modulation of lipid bilayer interfacial dipole potential by phloretin, RH421, and 6-ketocholestanol as probed by gramicidin channel conductance. Langmuir. 19:1439–1442. [Google Scholar]
- 12.Cladera, J., and P. O'Shea. 1998. Intramembrane molecular dipoles affect the membrane insertion and folding of a model amphiphilic peptide. Biophys. J. 74:2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cladera, J., I. Martin, J.-M. Ruysschaert, and P. O'Shea. 1999. Characterization of the sequence of interactions of the fusion domain of the simian immunodeficiency virus with membranes. J. Biol. Chem. 274:29951–29959. [DOI] [PubMed] [Google Scholar]
- 14.Maggio, B. 1999. Modulation of phospholipase A2 by electrostatic fields and dipole potential of glycosphingolipids in monolayers. J. Lipid Res. 40:930–939. [PubMed] [Google Scholar]
- 15.Alakoskela, J.-M. I., and P. K. J. Kinnunen. 2001. Control of a redox reaction on lipid bilayer surfaces by membrane dipole potential. Biophys. J. 80:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cladera, J., P. O'Shea, J. Hadgraft, and C. Valenta. 2003. Influence of molecular dipoles on human skin permeability: use of 6-ketocholestanol to enhance transdermal delivery of bacitracin. J. Pharm. Sci. 92:1018–1027. [DOI] [PubMed] [Google Scholar]
- 17.Starke-Peterkovic, T., N. Turner, P. L. Else, and R. J. Clarke. 2005. Electric field strength of membrane lipids from vertebrate species: membrane lipid composition and Na+,K+-ATPase molecular activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288:R663–R670. [DOI] [PubMed] [Google Scholar]
- 18.Alakoskela, J.-M. I., T. Söderlund, J. M. Holopainen, and P. K. J. Kinnunen. 2004. Dipole potential and head-group spacing are determinants for the membrane partitioning of pregnanolone. Mol. Pharmacol. 66:161–168. [DOI] [PubMed] [Google Scholar]
- 19.Asawakarn, T., J. Cladera, and P. O'Shea. 2001. Effects of the membrane dipole potential on the interaction of saquinavir with phospholipid membranes and plasma membrane receptor of caco-2 cells. J. Biol. Chem. 276:38457–38463. [DOI] [PubMed] [Google Scholar]
- 20.Luker, G. D., T. P. Flagg, Q. Sha, K. E. Luker, C. M. Pica, C. G. Nichols, and D. Piwnica-Worms. 2001. MDR1 P-glycoprotein reduces influx of substrates without affecting membrane potential. J. Biol. Chem. 276:49053–49060. [DOI] [PubMed] [Google Scholar]
- 21.O'Shea, P. 2003. Intermolecular interactions with/within cell membranes and the trinity of membrane potentials: kinetics and imaging. Biochem. Soc. Trans. 31:990–996. [DOI] [PubMed] [Google Scholar]
- 22.Andersen, O. S., and M. Fuchs. 1975. Potential energy barriers to ion transport within lipid bilayers. Studies with tetraphenylborate. Biophys. J. 15:795–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickar, A. D., and R. Benz. 1978. Transport of oppositely charged lipophilic probe ions in lipid bilayer membranes having various structures. J. Membr. Biol. 44:353–376. [Google Scholar]
- 24.Hladky, S., and D. A. Haydon. 1973. Membrane conductance and surface potential. Biochim. Biophys. Acta. 318:464–468. [DOI] [PubMed] [Google Scholar]
- 25.Smaby, J. M., and H. L. Brockman. 1990. Surface dipole moments of lipids at the argon-water interface. Similarities among glycerol-ester-based lipids. Biophys. J. 58:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabdoulline, R. R., G. Vanderkooi, and C. Zheng. 1996. Comparison of the structures of dimyristoylphosphatidylcholine in the presence and absence of cholesterol by molecular dynamics simulations. J. Phys. Chem. 100:15942–15946. [Google Scholar]
- 27.Hofsäβ, C., E. Lindahl, and O. Edholm. 2003. Molecular dynamics simulations of phospholipid bilayers with cholesterol. Biophys. J. 84:2192–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smondyrev, A. M., and M. L. Berkowitz. 2001. Effects of oxygenated sterol on phospholipid bilayer properties: a molecular dynamics simulation. Chem. Phys. Lipids. 112:31–39. [DOI] [PubMed] [Google Scholar]
- 29.Chiu, S. W., E. Jakobsson, and H. L. Scott. 2001. Combined Monte Carlo and molecular dynamics simulation of hydrated dipalmitoyl-phosphatidylcholine-cholesterol lipid bilayers. J. Chem. Phys. 114:5435–5443. [Google Scholar]
- 30.Gross, E., R. S. Bedlack Jr., and L. M. Loew. 1994. Dual-wavelength ratiometric fluorescence measurement of the membrane dipole potential. Biophys. J. 67:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke, R. J. 1997. Effect of lipid structure on the dipole potential of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 1327:269–278. [DOI] [PubMed] [Google Scholar]
- 32.Jørgensen, P. 1974. Purification and characterization of (Na+ + K+)-ATPase. III. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim. Biophys. Acta. 356:36–52. [DOI] [PubMed] [Google Scholar]
- 33.Folch, J., M. Less, and G. H. S. Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497–509. [PubMed] [Google Scholar]
- 34.Mills, G. L., P. A. Lane, and P. K. Weech. 1984. A guidebook to lipoprotein technique. In Laboratory Techniques in Biochemistry and Molecular Biology. R. H. Burdon and P. H. Knippenberg, editors. Elsevier Science, New York. 240–241.
- 35.Clarke, R. J., and D. J. Kane. 1997. Optical detection of membrane dipole potential: avoidance of fluidity and dye-induced effects. Biochim. Biophys. Acta. 1323:223–239. [DOI] [PubMed] [Google Scholar]
- 36.Gimpl, G., K. Burger, and F. Fahrenholz. 1997. Cholesterol as modulator of receptor function. Biochemistry. 36:10959–10974. [DOI] [PubMed] [Google Scholar]
- 37.Hansen, G. H., L.-L. Niels-Christiansen, E. Thorsen, L. Immerdal, and E. Michael Danielsen. 2000. Cholesterol depletion of enterocytes. Effect on the Golgi complex and apical membrane trafficking. J. Biol. Chem. 275:5136–5142. [DOI] [PubMed] [Google Scholar]
- 38.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–41. [DOI] [PubMed] [Google Scholar]
- 39.Marsh, D. 1990. Handbook of Lipid Bilayers. CRC Press, Boca Raton, FL.
- 40.Franks, N. P. 1976. Structural analysis of hydrated egg lecithin and cholesterol bilayers. I. X-ray diffraction. J. Mol. Biol. 100:345–358. [DOI] [PubMed] [Google Scholar]
- 41.McIntosh, T. J. 1978. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 513:43–58. [DOI] [PubMed] [Google Scholar]
- 42.Worcester, D. L., and N. P. Franks. 1976. Structural analysis of hydrated egg lecithin and cholesterol bilayers. II. Neutron diffraction. J. Mol. Biol. 100:359–378. [DOI] [PubMed] [Google Scholar]
- 43.Ramsammy, L. S., V. P. S. Chauhan, L. L. Box, and H. Brockerhoff. 1984. Interactions in the hydrogen belts of membranes: cholesterol leaving phosphatidylcholine bilayers. Biochem. Biophys. Res. Commun. 118:743–746. [DOI] [PubMed] [Google Scholar]
- 44.Demel, R. A., and B. De Kruyff. 1976. The function of sterols in membranes. Biochim. Biophys. Acta. 457:109–132. [DOI] [PubMed] [Google Scholar]
- 45.McClellan, A. L. 1963. Tables of Experimental Dipole Moments. W. H. Freeman and Company, San Francisco.
- 46.Flewelling, R. F., and W. L. Hubbell. 1986. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys. J. 49:541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almeida, P. F. F., W. L. C. Vaz, and T. E. Thompson. 1992. Lateral diffusion in liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: a free volume analysis. Biochemistry. 31:6739–6747. [DOI] [PubMed] [Google Scholar]
- 48.Tu, K., M. L. Klein, and D. J. Tobias. 1998. Constant-pressure molecular dynamics investigation of cholesterol effects in a dipalmitoylphosphatidylcholine bilayer. Biophys. J. 75:2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Bernard. 1958. Molecular associations between lipids. II. Lecithin and cholesterol. Bull. Soc. Chim. Biol. (Paris). 40:161–164. [PubMed] [Google Scholar]
- 50.Lecuyer, H., and D. G. Dervichian. 1969. Structure of lecithin and cholesterol. J. Mol. Biol. 45:39–57. [DOI] [PubMed] [Google Scholar]
- 51.Stockton, G. W., and I. C. P. Smith. 1976. A deuterium nuclear magnetic resonance study of the condensing effect of cholesterol on egg phosphatidylcholine bilayer membranes. I. Perdeuterated fatty acid probes. Chem. Phys. Lipids. 17:251–263. [DOI] [PubMed] [Google Scholar]
- 52.Blume, A. 1979. A comparative study of the phase transitions of phospholipid bilayers and monolayers. Biochim. Biophys. Acta. 557:32–44. [DOI] [PubMed] [Google Scholar]
- 53.Simon, S. A., and T. J. McIntosh. 1986. Depth of water penetration into lipid bilayers. Methods Enzymol. 127:511–521. [DOI] [PubMed] [Google Scholar]
- 54.Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]
- 55.Smondyrev, A. M., and M. L. Berkowitz. 1999. Structure of dipalmitoylphosphatidylcholine/cholesterol bilayer at low and high cholesterol concentrations: molecular dynamics simulation. Biophys. J. 77:2075–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker, A., K. Miles, K. H. Cheng, and J. Huang. 2004. Lateral distribution of cholesterol in dioleoylphosphatidylcholine lipid bilayers: cholesterol-phospholipid interactions at high cholesterol limit. Biophys. J. 86:1532–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chong, P. L.-G., and I. P. Sugár. 2002. Fluorescence studies of lipid regular distribution in membranes. Chem. Phys. Lipids. 116:153–175. [DOI] [PubMed] [Google Scholar]
- 58.Chong, P. L.-G., and M. Olsher. 2004. Fluorescence studies of the existence and functional importance of regular distributions in liposomal membranes. Soft Materials. 2:85–108. [Google Scholar]
- 59.Jedlovsky, P., N. Medvedev, and M. Mezei. 2004. Effect of cholesterol on the properties of phospholipid membranes. 3. Local lateral structure. J. Phys. Chem. B. 108:465–472. [DOI] [PubMed] [Google Scholar]
- 60.Shin, Y.-K., J. K. Moscicki, and J. H. Freed. 1990. Dynamics of phosphatidylcholine-cholesterol mixed model membranes in the liquid crystalline state. Biophys. J. 57:445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huster, D., K. Arnold, and K. Gawrisch. 1998. Influence of docosahexanoic acid and cholesterol on lateral lipid organization in phospholipid mixtures. Biochemistry. 37:17299–17308. [DOI] [PubMed] [Google Scholar]
- 62.Lund-Katz, S., H. M. Laboda, L. R. McLean, and M. C. Phillips. 1988. Influence of molecular packing and phospholipid type on rates of cholesterol exchange. Biochemistry. 27:3416–3423. [DOI] [PubMed] [Google Scholar]
- 63.Yeagle, P. L., J. Young, and D. Rice. 1988. Effects of cholesterol on (Na+,K+)-ATPase ATP hydrolyzing activity in bovine kidney. Biochemistry. 27:6449–6452. [DOI] [PubMed] [Google Scholar]
- 64.Sotomayor, C. P., L. F. Aguilar, F. J. Cuevas, M. K. Helms, and D. M. Jameson. 2000. Modulation of pig kidney Na+/K+-ATPase activity by cholesterol: role of hydration. Biochemistry. 39:10928–10935. [DOI] [PubMed] [Google Scholar]
- 65.Balut, C., P. Steels, M. Radu, M. Ameloot, W. Van Driessche, and D. Jans. 2006. Membrane cholesterol extraction decreases Na+ transport in A6 renal epithelia. Am. J. Physiol. Cell Physiol. 290:C87–C94. [DOI] [PubMed] [Google Scholar]
- 66.Cornelius, F. 2001. Modulation of Na,K-ATPase and Na-ATPase activity by phospholipids and cholesterol. I. Steady-state kinetics. Biochemistry. 40:8842–8851. [DOI] [PubMed] [Google Scholar]
- 67.Ganea, C., A. Babes, C. Lüpfert, E. Grell, K. Fendler, and R. J. Clarke. 1999. Hofmeister effects of anions on the kinetics of partial reactions of the Na+,K+-ATPase. Biophys. J. 77:267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kong, B. Y., and R. J. Clarke. 2004. Identification of potential regulatory sites of the Na+,K+-ATPase by kinetic analysis. Biochemistry. 43:2241–2250. [DOI] [PubMed] [Google Scholar]
- 69.Leonarduzzi, G., F. Biasi, E. Chiarpotto, and G. Poli. 2004. Trojan horse-like behavior of a biologically representative mixture of oxysterols. Mol. Aspects Med. 25:155–167. [DOI] [PubMed] [Google Scholar]