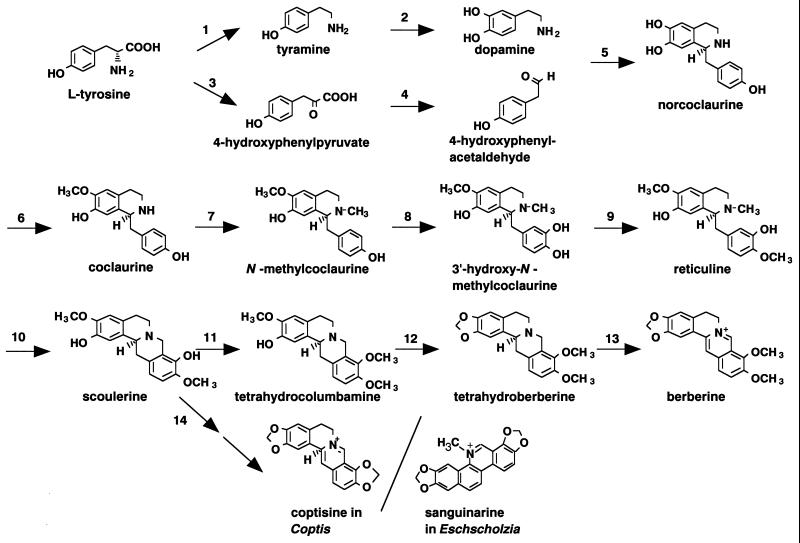
Figure 2.
Schematic biosynthetic pathway for a variety of isoquinoline alkaloids. 1, l-tyrosine decarboxylase; 2, phenolase; 3, l-tyrosine transaminase; 4, p-hydroxyphenylpyruvate decarboxylase; 5, (S)-norcoclaurine synthase; 6, (S)-adenosyl-l-methionine:norcoclaurine 6OMT; 7, (S)-adenosyl-l-methionine:coclaurine N-methyltransferase; 8, N-methylcoclaurine-4′-hydroxylase; 9, S-adenosyl-l-methionine: 3′-hydroxy-N-methylcoclaurine 4′OMT; 10, berberine bridge enzyme; 11, S-adenosyl-l-methionine:scoulerine 9-O-methyltransferase; 12, (S)-canadine synthase; 13, tetrahydroprotoberberine oxidase; 14, cheilanthifolin synthase.