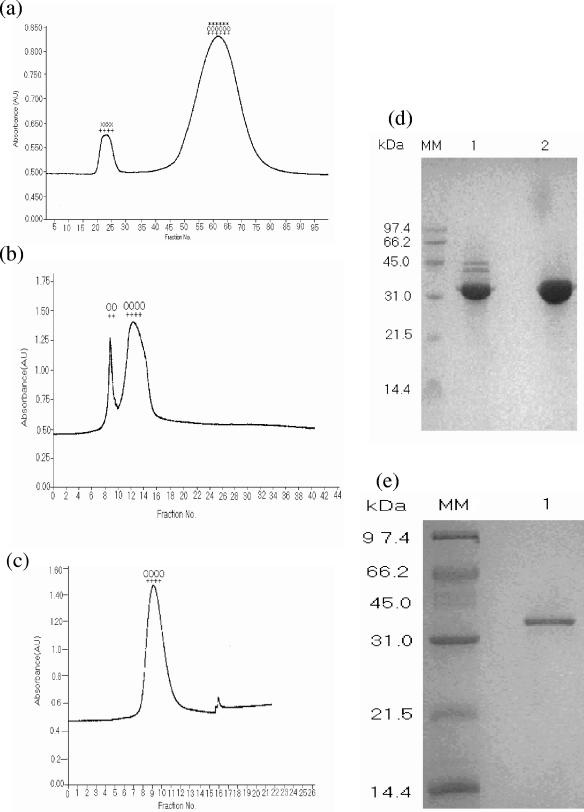
FIG. 1.
Chromatographic profiles showing purification of 35-kDa HAP from the culture supernatant of V. cholerae PL-21 strain. (a) Gel filtration chromatography column on G-200 column with ammonium sulfate-precipitated proteins. (b) Anion-exchange chromatography on DE-52 column of pooled fractions showing HA, protease, and LAP activity from G-200 column. (c) Gel filtration chromatography on G-75 column of pooled fractions of the second peak from DE-52 column. The presence of HA (++), protease (00), aminopeptidase (**), and lipopolysaccharide (xx) activity in the peaks is indicated. Absorbance is shown in arbitrary units (AU). (d) SDS-PAGE (12.5%) of the proteins from the two peaks in Fig. 1b. Lanes 1 and 2 contain the proteins from the first and second peaks, respectively. (e) SDS-PAGE (12.5%) of the protein from the single peak in Fig. 1c. The positions (in kilodaltons) of molecular mass markers (MM) are shown to the left of the gels.