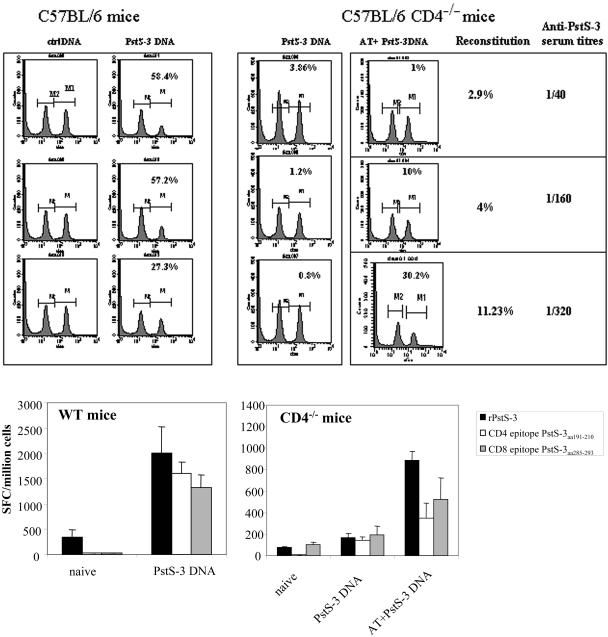
FIG. 4.
Analysis of in vivo CTL activity, antibody production, and in vitro IFN-γ production by CD8+ T cells in WT, CD4−/−, and reconstituted CD4−/− mice vaccinated with PstS-3 pDNA. (Top) CD4−/− mice, CD4−/− mice adoptively transferred with 2.5 × 106, 5 × 106, or 20 × 106 CD4+ T cells, and WT mice were vaccinated with 100 μg of PstS-3 DNA three times at 3-week intervals. One week after the third immunization, mice were analyzed for in vivo CTL activity. Syngeneic splenic T cells (20 × 106/mouse) were used as targets; of these, half were pulsed with the PstS-3 peptide (aa 285 to 293, SGVGNDLVL, 10 μg/ml) and labeled with CFSEHIGH and the other half were labeled with CFSELOW, as described in Materials and Methods, and a 1:1 mix was injected into the vaccinated hosts. After 20 h, mice were sacrificed, spleens were removed and made into single-cell suspensions, and cell counts were acquired on a cytofluorometer. A decrease in the peak of cells corresponding to peptide-pulsed and CFSEHIGH-labeled cells is indicative of specific in vivo CTL activity. Percentages represent CTL activity in each mouse compared to control DNA-vaccinated mice. In vivo CTL activity and anti-PstS-3 antibody titers in sera correlated with reconstitution of the CD4-T-cell compartment, as illustrated. (Bottom) Splenic cells from WT and CD4−/− mice before and after vaccination with PstS-3 pDNA, and after AT (20 × 106 CD4+ T cells) and vaccination in the case of CD4−/− mice, were tested for the capacity to produce IFN-γ in response to recombinant PstS-3 protein and its CD4+- and CD8+-T-cell peptides, aa 191 to 210 and aa 285 to 293, respectively. Data are from one representative experiment of three performed. SFC, spot-forming cells.