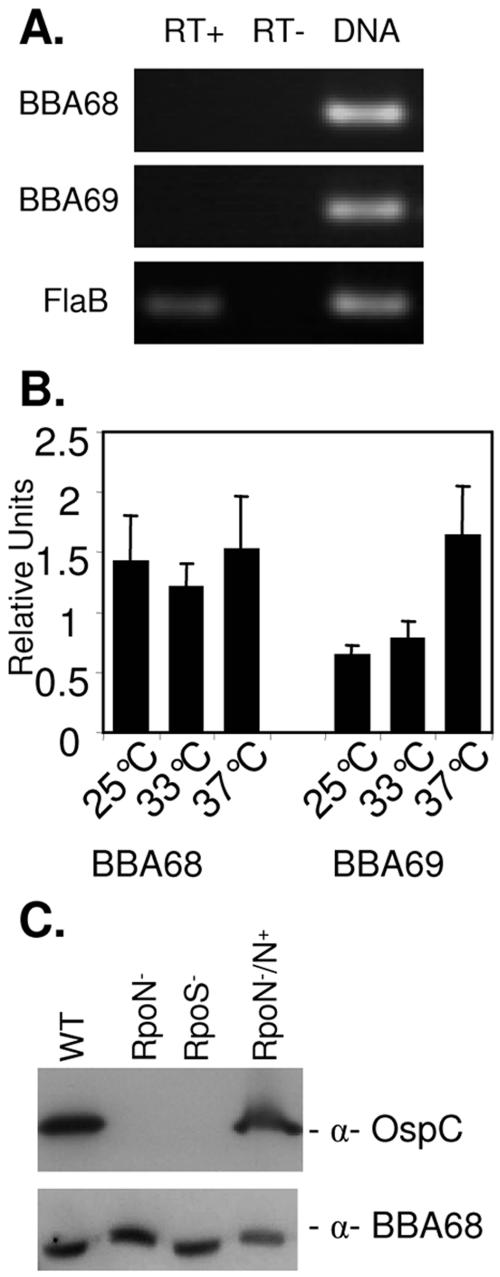
FIG. 3.
Real-time RT-PCR analysis of BBA68 and BBA69 gene transcription and analysis of the effect of RpoN and RpoS knockouts on protein production. RNA was isolated from infected mouse tissue and tested by real-time RT-PCR for BBA68 and BBA69 gene and flaB transcripts. Detection of flaB served as the positive control, and reactions run without reverse transcriptase served as the negative controls. As an additional positive control, the primers were also assessed for their ability to PCR amplify their corresponding target genes using genomic DNA as the template. After 40 cycles, an aliquot of each reaction mixture was analyzed by agarose gel electrophoresis and ethidium bromide staining (A). (B) Data from real-time RT-PCR analysis of BBA68 and BBA69 gene and flaB transcription in B. burgdorferi B31MI grown at either 25, 33, or 37°C in BSK-H complete media. Reactions were run in triplicate with two replicates, and the variance is indicated. RNA isolation and all associated methods were exactly as previously described (29). (C) Immunoblots of B. burgdorferi strain 297 in which RpoS or RpoN had been inactivated were screened with anti-BBA68 (α-BBA68) and anti-OspC antiserum (positive control). As controls, the wild type (WT) and the RpoN (RpoN−/N+) complemented strain were included on each blot.