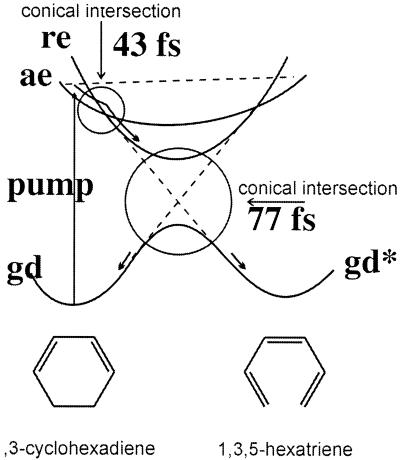
Figure 2.
A schematic drawing of the potentials for an ultrafast rearrangement that goes via a conical intersection. The time scales are experimental (14) from the kinetic simulation for the cyclohexadiene ring opening to hexatriene. The cyclohexadiene/hexatriene electrocyclic ring opening, occurring in less than 100 fs, may be considered as a prototype of a molecular ultrafast switch. The optical excitation goes via the direct access of a bright state, ae, which reacts by going first to a dark (photoreactive) excited state, re, and then to the product electronic ground state, gd*. For the cyclohexadiene system one can readily convert about 0.1% of all molecules in a sample to the excited state. The required molecular bistability in the ground state mediated by electronically excited states can be found also in related molecular systems (electrocyclic reactions, cycloadditions, sigmatropic shifts, tautomers, change in spin systems, electron transfer/redox photochemistry, etc.) (see also Figs. 4 and 5 and ref. 16).