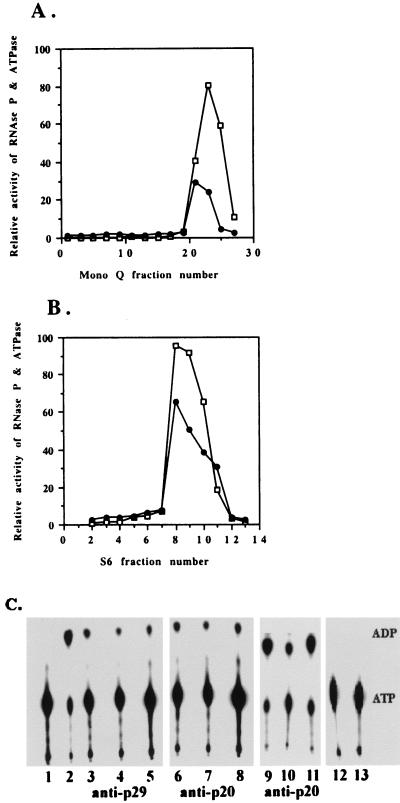
Figure 1.
Nuclear RNase P purified from HeLa cells has ATPase activity. The RNase P (□) and ATPase (●) activities of the fractions from a Mono Q FPLC column (A) and an S6 column (B) were assayed as described in Materials and Methods. (C) Immunoprecipitation of ATPase activities by polyclonal rabbit antibodies against recombinant Rpp29 and Rpp20 protein subunits of human RNase P. The procedure used is described in Materials and Methods. Lane 1, Incubation of ATP alone. Lane 2, Addition of RNase P from an S6 column. Lanes 3–5, Immunoprecipitation of human RNase P with Rpp29 antibody and (lanes 6–8) with Rpp20 antibody. Lanes 9–11, Immunoprecipitation of Rpp20 with Rpp20 antibody. Lane 12, Rpp29 antibody with no addition of RNase P. Lane 13, Rpp20 antibody with no addition of RNase P. Lanes 3, 6, and 9, aliquots from initial supernatants. Lanes 4, 7, and 10, aliquots from supernatants after overnight immunoprecipitation. Lanes 5, 8, and 11, aliquots from the pellets. The sum of ATPase activity recovered after immunoprecipitation is somewhat greater than that found in the initial supernatant fraction both because of the higher efficiency of the ATPase activity in the undiluted 1× ATPase buffer used to assay the immunoprecipitated fractions and the fact that even rabbit preimmune control serum enhances ATPase activity.