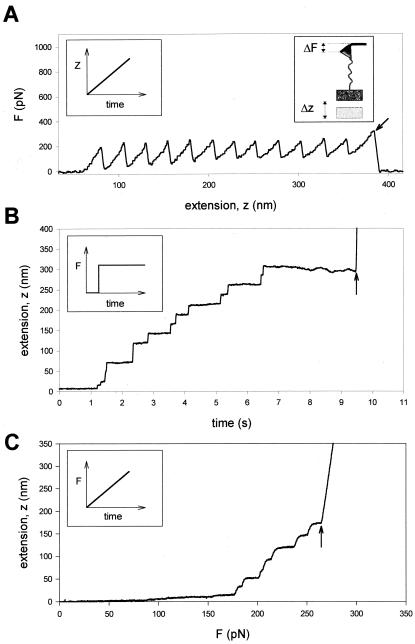
Figure 2.
Comparison of single protein unfolding events captured with an AFM in length-clamp mode (A) and a force-clamp mode (B and C). (A) In standard mode AFM, the positioner is moved linearly as a function of time (Left Inset) and the pulling force is measured from the degree of deflection of the cantilever (Right Inset). When a modular protein is stretched between the cantilever tip and a coverslip attached to a piezo-electric positioner, the resulting force-extension curve shows a sawtooth pattern with equally spaced force peaks, where each force peak represents the unfolding of single protein domains. The recording corresponds to the stretching and unfolding of a polyprotein containing 12 identical repeats of titin Ig domain I27. The last peak corresponds to the detachment of the protein from the cantilever and the measured force drops to zero (arrow). (B and C) In constant force AFM, the force applied on the protein is kept at a set value by using a force-clamp system that continuously changes the position of the piezoelectric device. (B) When a step increase in force is applied to a single I27 polyprotein, the extension-time curve shows step increases in the length of the protein, where each step is about 22 nm in size. This trace was obtained by stepping the force from −400 to 180 pN. (C) Under force-clamp conditions the force applied to a single I2712 polyprotein is increased linearly with time. The figure shows the resulting length versus force trace. As shown, stepwise unfolding events occur over a narrow range of forces (in this case ≈170–250 pN) and a much shorter time (≈2 s) than those observed under constant force. In these experiments the steps are slightly rounded because of the limited frequency response of the feedback system. In both cases (B and C), the last step increase in length marks the detachment of the protein from the cantilever, where the position of the piezoelectric actuator increases rapidly to saturation (arrows).