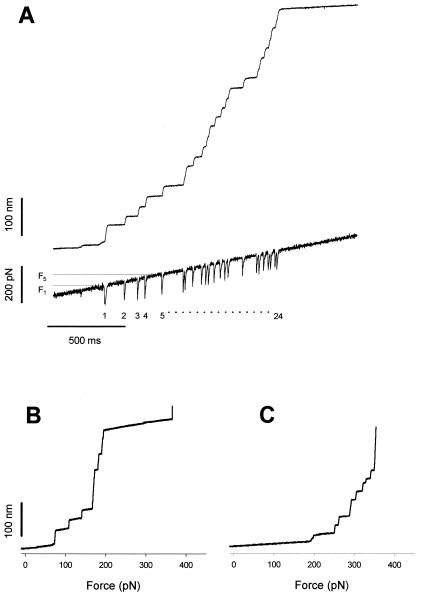
Figure 5.
Measurements of the force dependence of the unfolding probability using a force ramp. Force-ramp experiments apply a linearly increasing force to a folded protein causing the full unfolding of its modules under well-defined conditions and over a relatively short period. (A) Stepwise unfolding of a native cardiac titin molecule using the force-ramp method. The upper trace shows that the molecule elongates in 24 steps over a ≈200 pN range. The lower trace shows the time course of the force. The downward transients are caused by the feedback lag and serve as useful markers of unfolding. (B) Stepwise unfolding events for the I2712 polyprotein recorded under conditions similar to those shown in A. The step increases in length are plotted as a function of the applied force. (C) A similar experiment done with the I288 polyprotein shows that this polyprotein unfolds in a higher force range than the I2712 polyprotein.