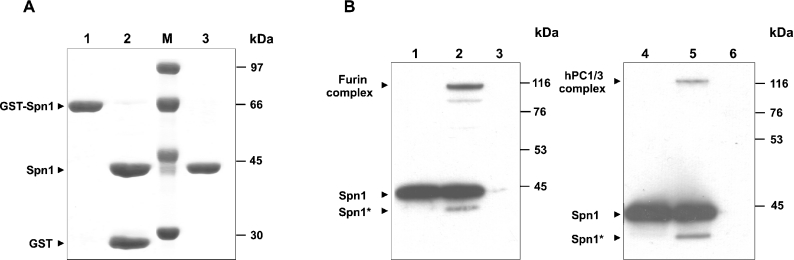
Figure 2. Isolation of recombinant Spn1 and analysis of complex formation with PCs.
(A) GST-fused Spn1 was purified from bacterial extracts via glutathione–Sepharose™ 4B (lane 1). After treatment with TEV protease (lane 2), His-tagged contaminants were removed by metal chelate affinity chromatography (lane 3). Proteins were stained with Coomassie Brilliant Blue after SDS/PAGE (10% gels). The positions of molecular mass markers (lane M) are given on the right. (B) Purified Spn1 was incubated with human furin or with hPC1/3 respectively. After SDS/PAGE (10% gels) under reducing conditions, the reaction products were blotted and analysed with antibodies directed against Spn1. Lanes 1 and 4, Spn1; lane 2, Spn1 incubated with furin; lane 3, furin; lane 5, Spn1 incubated with hPC1/3; lane 6, hPC1/3. Cleaved forms of Spn1 are marked by an asterisk. The signal at approx. 100 kDa in lane 2 is presumed to represent a partially degraded Spn1–furin complex. The positions and molecular masses of marker proteins are indicated on the right.